Trainee Specialist Pharmacovigilance Job in Noida at Cencora for graduates in pharmacy and life sciences with safety reporting experience.
About the Company
Cencora is a leading global healthcare organization dedicated to creating healthier futures for people and animals worldwide. Through innovation, compliance excellence, and patient safety leadership, Cencora supports pharmaceutical, biotechnology, and healthcare partners across the entire product lifecycle. Trainee Specialist Pharmacovigilance Job in Noida
In India, this opportunity is offered through its affiliated company, PharmaLex India Private Limited, a well-recognized name in pharmacovigilance, regulatory affairs, and risk management services. The organization provides strong career foundations for freshers and early professionals in drug safety and epidemiology. Trainee Specialist Pharmacovigilance Job in Noida
Job Details
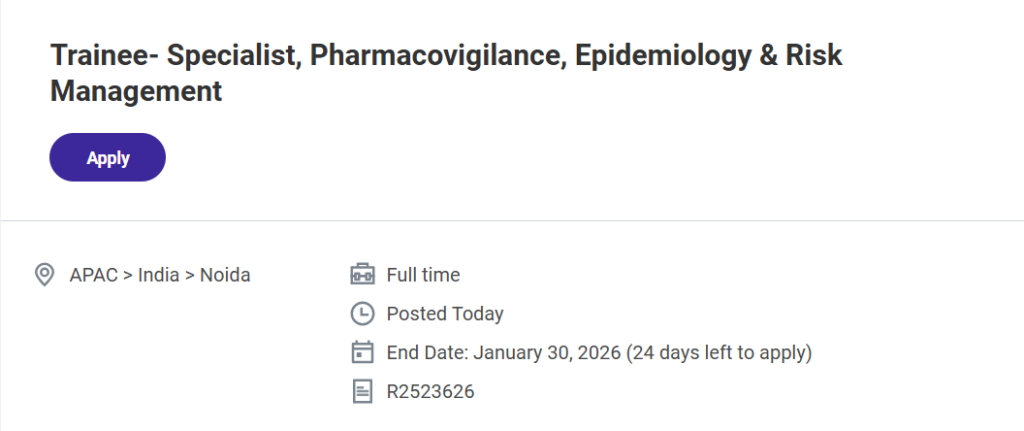
- Job Title: Trainee – Specialist, Pharmacovigilance, Epidemiology & Risk Management
- Location: Noida, India (APAC Region)
- Employment Type: Full Time
- Job Requisition ID: R2523626
- Application Deadline: January 30, 2026
Job Description
Cencora is seeking motivated and detail-oriented graduates to join its Pharmacovigilance and Risk Management team as a Trainee Specialist. This role is ideal for candidates looking to start or strengthen their career in drug safety, adverse event reporting, and regulatory compliance within a global healthcare environment. Trainee Specialist Pharmacovigilance Job in Noida
The position involves hands-on exposure to global pharmacovigilance processes, regulatory reporting standards, and safety database management while working under experienced supervisors and quality-driven systems. Trainee Specialist Pharmacovigilance Job in Noida
Key Responsibilities
- Perform triage and initial validity assessments of adverse event cases from multiple sources
- Process spontaneous reports, clinical trial cases, health authority notifications, and literature cases
- Conduct accurate and timely data entry into pharmacovigilance safety databases
- Complete initial Individual Case Safety Report (ICSR) assessments per regulatory requirements
- Evaluate cases for expedited reporting obligations and regulatory timelines
- Prepare regulatory reporting forms such as CIOMS I, MedWatch, and XML submissions
- Draft and send follow-up queries to obtain missing or additional safety information
- Ensure complete and compliant case documentation according to quality standards
- Conduct ICSR searches and retrieval from the EVWEB database
- Perform company and non-company case assessments
- Support additional pharmacovigilance tasks assigned by the supervisor
Trainee Specialist Pharmacovigilance Job in Noida
Skills / Qualifications
Educational Qualification
- B.Pharm
- M.Pharm
- BDS
- Life Science Graduate
Required Skills
- Strong written and verbal communication skills
- High attention to detail and data accuracy
- Ability to work under strict regulatory timelines
- Basic understanding of pharmacovigilance concepts (preferred)
- Willingness to learn global safety reporting standards
Benefits / Perks
- Full-time employment with structured training
- Exposure to global pharmacovigilance systems and clients
- Career growth in drug safety, epidemiology, and risk management
- Work with international regulatory standards and processes
- Inclusive and equal opportunity workplace
- Reasonable accommodation support for individuals with disabilities
Benefits may vary based on local market practices and employment policies. Trainee Specialist Pharmacovigilance Job in Noida
Why You Should Join
- Work with a globally respected healthcare organization
- Build a long-term career in pharmacovigilance and regulatory safety
- Gain hands-on experience in ICSR processing and safety reporting
- Learn from industry experts in a compliance-driven environment
- Opportunity to grow within Cencora’s global healthcare network
This role is especially suitable for freshers and early-career professionals aiming to enter pharmacovigilance and drug safety domains. Trainee Specialist Pharmacovigilance Job in Noida
How to Apply
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.
FAQs
Q1. Is this job suitable for freshers?
Yes, this trainee role is ideal for freshers and early professionals with pharmacy or life science backgrounds.
Q2. Is pharmacovigilance experience mandatory?
No, prior experience is preferred but not mandatory. Training will be provided.
Q3. What is the job location?
The position is based in Noida, India.
Q4. Is this a permanent job?
Yes, this is a full-time role.
Q5. What is the last date to apply?
January 30, 2026.



