QA Production Engineering Jobs at Eugia Pharma professionals in Telangana through walk-in interview for experienced injectable manufacturing candidates.
About the Company
Eugia Pharma Specialities Limited is a leading pharmaceutical company specializing in injectable formulations and specialty generic products. As part of the Aurobindo Pharma Group, Eugia Pharma has established a strong presence in global regulated markets through its focus on quality-driven manufacturing, regulatory compliance, and innovation in parenteral dosage forms.
The company operates advanced manufacturing facilities equipped with modern aseptic processing lines, lyophilizers, BFS technology, and fully automated instrumentation systems. Eugia Pharma is widely recognized for producing high-quality sterile injectables that comply with USFDA, EU-GMP, and other international regulatory standards. QA Production Engineering Jobs at Eugia Pharma
With continuous capacity expansion and increasing demand for injectable products worldwide, Eugia Pharma is strengthening its operations team across Quality Assurance, Production, and Engineering departments. This walk-in interview drive is aimed at experienced professionals seeking long-term growth opportunities in a reputed and professionally managed pharmaceutical organization. QA Production Engineering Jobs at Eugia Pharma
Job Details
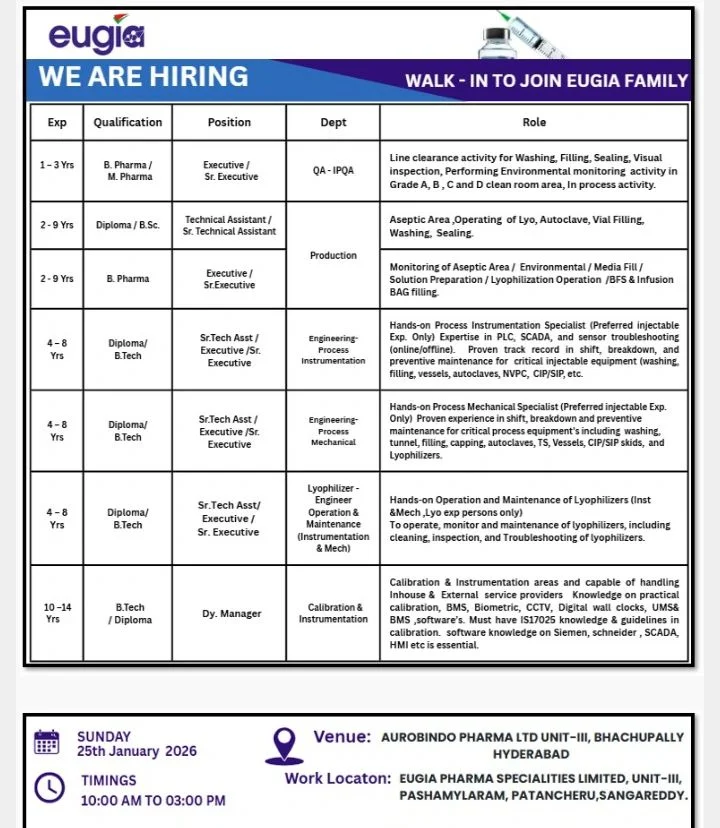
- Job Title: QA, Production, Engineering Roles
- Company Name: Eugia Pharma Specialities Limited
- Group: Aurobindo Pharma Group
- Departments: Quality Assurance, Production, Engineering, Calibration & Instrumentation
- Job Location: Telangana
- Manufacturing Unit: Unit-III, Pashamylaram, Patancheru, Sangareddy, Hyderabad
- Employment Type: Full-Time
- Experience Required: 1 to 14 Years
- Education Qualification: Diploma, B.Sc, B.Pharm, M.Pharm, B.Tech
- Job Type: Walk-In Interview
- Interview Date: Sunday, 25 January 2026
- Interview Time: 10:00 AM to 3:00 PM
- Job Status: Verified Job
Job Description
Eugia Pharma is conducting a walk-in interview to recruit skilled and experienced professionals for its injectable manufacturing operations. The hiring covers multiple technical and managerial roles across QA, Production, Engineering (Instrumentation and Mechanical), Lyophilizer Operations, and Calibration departments. QA Production Engineering Jobs at Eugia Pharma
The roles require hands-on experience in sterile injectable manufacturing environments, including aseptic processing, environmental monitoring, equipment operation, maintenance, and GMP documentation. Selected candidates will be involved in critical manufacturing and quality activities that directly impact product safety, regulatory compliance, and operational efficiency. QA Production Engineering Jobs at Eugia Pharma
This opportunity is ideal for professionals who have previously worked in regulated injectable facilities and are looking to advance their careers with a well-established pharmaceutical organization offering stability, growth, and exposure to advanced manufacturing technologies. QA Production Engineering Jobs at Eugia Pharma
Skills and Qualifications
Educational Qualifications
- Diploma in Engineering (Mechanical, Instrumentation, Electrical)
- B.Sc (Science or relevant discipline)
- B.Pharm or M.Pharm
- B.Tech (Mechanical, Instrumentation, Electrical, or related fields)
Experience Requirements
- Minimum 1 year and up to 14 years of relevant pharmaceutical manufacturing experience
- Experience in injectable or sterile manufacturing facilities is strongly preferred
Technical and Professional Skills
- Strong understanding of aseptic operations and cleanroom practices
- Knowledge of GMP documentation and regulatory compliance
- Hands-on experience with injectable production equipment
- Ability to work in shifts and handle shop-floor responsibilities
- Troubleshooting and preventive maintenance skills for engineering roles
QA Production Engineering Jobs at Eugia Pharma
Key Responsibilities
Quality Assurance – IPQA (Executive / Senior Executive)
- Perform line clearance activities in sterile manufacturing areas
- Monitor washing, filling, sealing, and visual inspection processes
- Conduct environmental monitoring in Grade A, B, C, and D cleanrooms
- Ensure compliance with SOPs and GMP requirements
- Support deviation handling and quality documentation
Production – Technical Assistant / Senior Technical Assistant
- Operate aseptic manufacturing equipment in injectable areas
- Handle lyophilizers, autoclaves, vial washing, filling, and sealing machines
- Follow aseptic gowning and cleanroom procedures
- Support routine production activities and documentation
Production – Executive / Senior Executive
- Monitor aseptic processing operations
- Perform environmental monitoring and media fill activities
- Handle infusion and solution preparation
- Operate lyophilization, BFS, and injectable bag filling lines
- Ensure adherence to production schedules and quality standards
QA Production Engineering Jobs at Eugia Pharma
Engineering – Process Instrumentation
- Maintain and troubleshoot online and offline instrumentation systems
- Work with PLC, SCADA, and HMI-based control systems
- Handle critical equipment such as filling machines, autoclaves, vessels, NPC, CIP/SIP systems
- Support breakdown maintenance and preventive maintenance programs
Engineering – Process Mechanical
- Perform mechanical maintenance of filling, capping, autoclaves, and lyophilizers
- Maintain tunnels, vessels, and CIP/SIP skids
- Support shift operations and equipment troubleshooting
- Ensure mechanical systems comply with GMP requirements
Lyophilizer Engineer – Instrumentation & Mechanical
- Operate and maintain lyophilizers
- Perform cleaning, inspection, and documentation activities
- Handle batch records and equipment logbooks
- Support qualification and validation activities
Deputy Manager – Calibration and Instrumentation
- Manage in-house and external calibration activities
- Maintain calibration schedules and documentation
- Handle systems such as BMS, biometric access, digital clocks, and UMS software
- Work with Siemens-based SCADA and HMI systems
- Ensure compliance with regulatory and audit requirements
QA Production Engineering Jobs at Eugia Pharma
Benefits and Perks
Eugia Pharma offers competitive compensation packages aligned with industry standards and experience levels. QA Production Engineering Jobs at Eugia Pharma
Estimated Salary Range
- Entry Level (1–3 years): ₹3.0 – ₹5.0 Lakhs per annum
- Mid Level (4–9 years): ₹4.5 – ₹8.0 Lakhs per annum
- Senior Level (10+ years): ₹8.0 – ₹15.0 Lakhs per annum or higher
Additional Benefits
- Provident Fund (PF)
- Employee State Insurance (ESI)
- Health insurance coverage
- Performance-based incentives
- Long-term career growth opportunities
- Exposure to regulated global markets
Why You Should Join Eugia Pharma
Eugia Pharma provides a strong platform for professionals seeking growth in injectable pharmaceutical manufacturing. Being part of the Aurobindo Pharma Group, the company offers stability, advanced infrastructure, and exposure to international regulatory standards.
Employees benefit from hands-on experience with modern aseptic technologies, lyophilization systems, and automated instrumentation. The organization promotes continuous learning, internal growth, and performance-based career progression. Working at Eugia Pharma allows professionals to contribute meaningfully to life-saving injectable medicines while building a rewarding long-term career.
FAQs
Who can attend this walk-in interview?
Candidates with 1 to 14 years of experience in injectable or pharmaceutical manufacturing can attend.
Is injectable experience mandatory?
Yes, experience in injectable or sterile manufacturing environments is strongly preferred for most roles.
Are freshers eligible?
This hiring drive is mainly for experienced professionals; freshers are generally not eligible.
Will selected candidates work in shifts?
Yes, most roles involve shift-based operations depending on department requirements.
Is this a permanent position?
Yes, all roles are full-time permanent positions based on company policies.
QA Production Engineering Jobs at Eugia Pharma
How to Apply
Interested candidates should attend the walk-in interview directly as per the details below:
Date: Sunday, 25 January 2026
Time: 10:00 AM to 3:00 PM
Interview Venue:
Aurobindo Pharma Ltd
Unit-III, Bhachupally
Hyderabad, Telangana
Selected candidates will be deployed at Eugia Pharma Specialities Limited, Unit-III, Pashamylaram, Patancheru, Sangareddy, Hyderabad, Telangana.
Documents to Carry:
- Updated resume
- Educational certificates
- Experience letters
- Recent passport-size photographs
Candidates are advised to arrive early, as walk-ins are conducted on a first-come, first-served basis. This is an excellent opportunity to join a reputed injectable pharmaceutical company and advance your career in quality, production, or engineering functions. QA Production Engineering Jobs at Eugia Pharma
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



