Apply online for ICON plc Hiring for Clinical Research Associate 1, India. Remote job opportunity for experienced clinical research professionals.
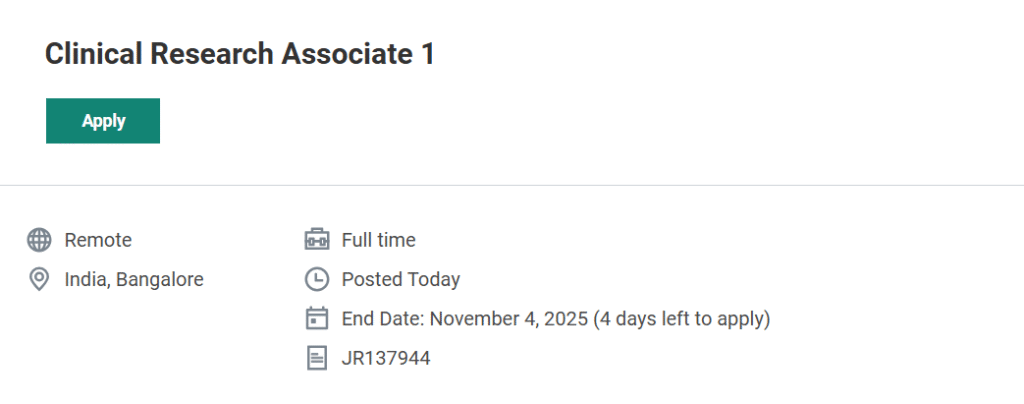
Job Title: Clinical Research Associate 1
Company: ICON plc
Location: India (Remote / Bangalore)
Experience: 1–4 Years
Employment Type: Full Time, Permanent
Industry: Clinical Research / Healthcare Intelligence
Last Date to Apply: November 4, 2025
Mode of Application: Online
About ICON plc
ICON plc is a world-leading healthcare intelligence and clinical research organization, recognized globally for innovation, quality, and excellence in clinical development. With operations in over 50 countries and a workforce dedicated to improving global health, ICON plays a vital role in helping pharmaceutical, biotechnology, and medical device companies bring new treatments to market. ICON plc Hiring for Clinical Research Associate 1
The company’s mission is clear — to transform healthcare through data-driven insights, patient-centered research, and operational excellence. ICON’s inclusive culture fosters professional growth, collaboration, and continuous learning, making it one of the most sought-after employers in the global life sciences industry. ICON plc Hiring for Clinical Research Associate 1
Position Overview: Clinical Research Associate 1
ICON plc is currently seeking a Clinical Research Associate (CRA 1) to join its expanding India-based remote research team. This position offers a unique opportunity to contribute to groundbreaking clinical studies, ensuring compliance with global regulatory standards while enhancing patient safety and clinical outcomes. ICON plc Hiring for Clinical Research Associate 1
The CRA will be responsible for monitoring clinical trials, coordinating study operations, and ensuring the quality and accuracy of data collection. Working within ICON’s innovative and flexible environment, you will collaborate with experienced professionals and global stakeholders to ensure clinical excellence. ICON plc Hiring for Clinical Research Associate 1
Key Responsibilities
As a Clinical Research Associate 1 at ICON plc, your responsibilities will include:
- Independent Monitoring: Manage all aspects of clinical trial setup, conduct, and closeout, ensuring adherence to study protocols, GCP, and regulatory requirements.
- Site Management: Actively coordinate with clinical sites, investigators, and site staff to maintain study timelines and ensure data accuracy.
- Documentation and Reporting: Maintain comprehensive study documentation, complete accurate status reports, and ensure proper tracking of clinical data.
- Query Resolution: Efficiently run sponsor-generated queries and address site-related issues to maintain data integrity.
- Cost and Quality Oversight: Take accountability for study cost efficiency while maintaining the highest quality standards.
- Feasibility Studies: Participate in the preparation and review of feasibility studies for new project proposals as required.
- Stakeholder Collaboration: Build and maintain strong, professional relationships with key stakeholders, clinical investigators, and project managers.
- Regulatory Compliance: Ensure that patient safety and data protection are maintained in accordance with ICON’s procedures, ICH-GCP guidelines, and sponsor expectations.
Educational Qualification and Experience
To qualify for the Clinical Research Associate 1 role at ICON plc, candidates must meet the following educational and professional requirements: ICON plc Hiring for Clinical Research Associate 1
- Education:
A university degree in Medicine, Science, Pharmacy, or related Life Science discipline is required. Equivalent qualifications with relevant experience will also be considered. - Experience:
- Minimum 1 to 4 years of experience in on-site monitoring or related clinical research roles.
- Strong working knowledge of ICH-GCP guidelines, clinical trial processes, and regulatory documentation.
- Previous experience in monitoring patient recruitment, data collection, and site management preferred.
Key Skills and Competencies
ICON plc is looking for candidates who bring technical expertise, precision, and a commitment to excellence. The following skills and competencies are essential for success in this role: ICON plc Hiring for Clinical Research Associate 1
- Regulatory Knowledge: Deep understanding of Good Clinical Practice (GCP), ICH guidelines, and FDA/EMA regulations.
- Analytical Thinking: Ability to evaluate and interpret medical data effectively.
- Communication Skills: Excellent written and verbal communication abilities to interact with investigators, clients, and internal teams.
- Problem-Solving: Proactive in identifying issues and providing timely solutions.
- Adaptability: Capable of managing multiple studies under tight timelines while ensuring data quality.
- Travel Readiness: Willingness to travel up to 60% of the time (both domestic and international) as per project needs.
- Technical Proficiency: Familiarity with clinical trial software systems and digital data collection tools.
Work Environment and Culture
At ICON plc, employees work in a collaborative, inclusive, and technology-driven environment. The company’s leadership believes in nurturing talent and promoting innovation. ICON plc Hiring for Clinical Research Associate 1
ICON’s core values — Integrity, Collaboration, Agility, and Inclusion — drive every aspect of its culture. Team members are encouraged to think innovatively, take ownership, and make meaningful contributions that enhance patient outcomes globally.
As a remote team member based in India, you will enjoy flexible work options while staying connected with global project teams through cutting-edge communication platforms.
Employee Benefits and Rewards
ICON plc values its employees and offers a comprehensive range of benefits designed to support professional growth, personal well-being, and work-life balance. ICON plc Hiring for Clinical Research Associate 1
Some of the benefits include:
- Competitive Salary: Industry-leading compensation structure based on qualifications and experience.
- Health and Wellness: Access to multiple health insurance options for employees and dependents.
- Annual Leave: Generous annual and sick leave entitlements.
- Retirement Plans: Competitive retirement savings and pension schemes to secure your financial future.
- Employee Assistance Programme (EAP): 24-hour access to a global network of over 80,000 health and wellness professionals through TELUS Health.
- Life Assurance: Comprehensive coverage ensuring financial protection for your loved ones.
- Work-Life Balance: Flexible benefits, including childcare vouchers, gym discounts, and travel subsidies.
- Career Growth: Learning and development programs for continued professional advancement within the organization.
Diversity, Inclusion, and Equal Opportunity
ICON plc is deeply committed to diversity, equity, and inclusion. The organization ensures a workplace that is free from discrimination and harassment, valuing the unique perspectives and backgrounds of every team member. ICON plc Hiring for Clinical Research Associate 1
All qualified applicants will receive equal consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, disability, or veteran status.
Candidates who require accommodations during the recruitment process or while performing job functions can request assistance at any time.
Why Join ICON plc?
Joining ICON plc means becoming part of a company that is transforming the landscape of clinical research and healthcare analytics. ICON plc Hiring for Clinical Research Associate 1
You’ll work alongside world-class professionals, participate in global clinical trials, and gain exposure to innovative digital solutions shaping the future of medical science.
Whether you are beginning your journey in clinical research or seeking to advance your career, ICON provides an environment that supports continuous learning, professional development, and impactful work. ICON plc Hiring for Clinical Research Associate 1
Job Summary Table
| Field | Details |
|---|---|
| Job Title | Clinical Research Associate 1 |
| Company | ICON plc |
| Industry | Healthcare Intelligence / Clinical Research |
| Experience Required | 1–4 Years |
| Qualification | Degree in Medicine, Science, or equivalent |
| Job Type | Full Time, Permanent |
| Location | Remote (India / Bangalore) |
| Last Date to Apply | November 4, 2025 |
| Application Mode | Online |
| Official Website | www.iconplc.com |
How to Apply
Interested candidates who meet the eligibility criteria can apply directly through the ICON Careers Portal. ICON plc Hiring for Clinical Research Associate 1
Follow the steps below to submit your application:
- Visit the official ICON careers page at https://careers.iconplc.com.
- Search for the role “Clinical Research Associate 1 – India (Remote)” using the job ID JR137944.
- Review the complete job description and eligibility requirements.
- Click on “Apply Now” to begin your application process.
- Fill out the online form with your updated details and upload your resume.
- Submit your application before the deadline – November 4, 2025.
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.
for delivering Latest Genuine Pharma Job Updates.
