ICON plc Hiring Clinical Data Coordinator I in India. Apply now for full-time positions in Bangalore, Trivandrum, and Chennai.
ICON plc Clinical Data Coordinator I Recruitment 2025 – Apply Online
ICON plc, one of the world’s leading healthcare intelligence and clinical research organizations, has announced an excellent opportunity for candidates looking to build their careers in clinical data management. The company is inviting applications for the position of Clinical Data Coordinator I (CDC I) for its offices located in Bangalore, Trivandrum, and Chennai. This full-time position offers a flexible work environment, competitive compensation, and a chance to work on groundbreaking global clinical trials. ICON plc Hiring Clinical Data Coordinator I
ICON plc is known for its innovation, integrity, and excellence in the field of clinical development. The company collaborates with major pharmaceutical, biotechnology, and medical device firms to bring new therapies and treatments to the market faster. Working at ICON means joining a dynamic global team dedicated to improving the lives of patients through scientific and data-driven advancements. ICON plc Hiring Clinical Data Coordinator I
Job Overview: Clinical Data Coordinator I (CDC I)
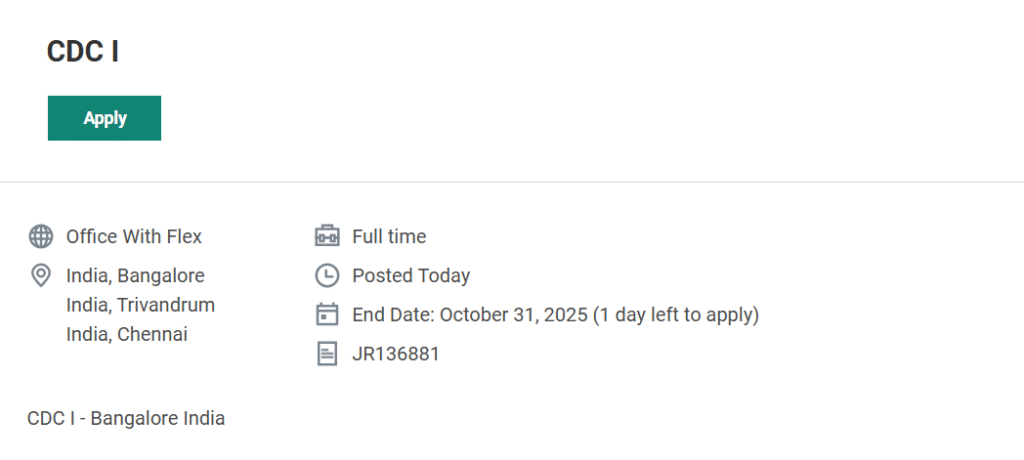
Position Title: Clinical Data Coordinator I
Job Location: Bangalore, Trivandrum, Chennai (Office with Flex)
Job Type: Full-Time
Department: Clinical Data Management
Application Deadline: October 31, 2025
Job Requisition ID: JR136881
As a Clinical Data Coordinator I, you will be part of ICON’s dedicated data management team responsible for ensuring high-quality data collection, validation, and reporting for global clinical trials. The role involves working closely with cross-functional teams to design, develop, and maintain electronic data capture (EDC) systems and ensure that clinical trial data meets regulatory and scientific standards. ICON plc Hiring Clinical Data Coordinator I
Key Responsibilities
The Clinical Data Coordinator I plays a vital role in the success of clinical trials by ensuring accurate, complete, and validated data. Some of the primary responsibilities include: ICON plc Hiring Clinical Data Coordinator I
- Assist in Study Design and Setup
- Support the Data Management Study Lead (DMSL) in developing electronic Case Report Forms (eCRFs), Data Validation Specifications (DVS), and Study-Specific Procedures (SSP).
- Collaborate with study teams to define database structures and validation rules.
- Data Review and Query Management
- Review clinical and third-party data according to the edit check specifications and data review plans.
- Generate clear, accurate, and concise queries to investigator sites to resolve discrepancies in data entries.
- Database Maintenance and Documentation
- Maintain accurate records of data management activities including Trial Master File (TMF) documentation.
- Ensure timely database updates, audits, and validation checks according to project timelines.
- Collaboration and Communication
- Communicate effectively with clinical data scientists, study leads, and other cross-functional team members.
- Provide status updates, raise potential issues, and suggest process improvements.
- Project Support Activities
- Participate in other project-related activities, such as filing, documentation, and dispatching queries, ensuring that study timelines are achieved.
- Support process improvement initiatives and contribute to quality control measures.
Required Qualifications and Experience
To be eligible for the Clinical Data Coordinator I position at ICON plc, candidates must meet the following criteria: ICON plc Hiring Clinical Data Coordinator I
- Educational Qualification:
Bachelor’s degree in Life Sciences, Pharmacy, Biotechnology, Healthcare, or a related field is mandatory. - Professional Experience:
Minimum 1.5 years of experience in Clinical Data Management (CDM) is required, including experience in data review, query management, database design, and TMF documentation. - Technical Expertise:
- Hands-on experience with Electronic Data Capture (EDC) systems such as Medidata, Oracle RDC, or similar platforms.
- Familiarity with data cleaning, coding, and validation tools.
- Regulatory Knowledge:
Understanding of ICH-GCP guidelines and other relevant data management standards is highly desirable. - Soft Skills:
- Strong attention to detail and commitment to data accuracy.
- Excellent verbal and written communication skills.
- Ability to work collaboratively in a fast-paced, team-oriented environment.
Why Join ICON plc?
At ICON plc, employees are the heart of innovation and success. The organization values diversity, inclusion, and continuous professional growth. By joining ICON, you become part of a global network of professionals dedicated to advancing healthcare research through data-driven excellence. ICON plc Hiring Clinical Data Coordinator I
Here’s what you can expect when you join the ICON team:
- Competitive Compensation and Benefits
ICON offers an attractive salary package along with performance-based incentives and recognition programs. - Comprehensive Health Insurance
Multiple health insurance plans are available to support the well-being of employees and their families. - Retirement Planning
Competitive retirement and savings options help employees plan confidently for their future. - Work-Life Balance
With flexible working options, including hybrid and office-flex arrangements, ICON ensures a balance between work and personal life. - Employee Assistance Programme (EAP)
ICON provides access to TELUS Health, a global assistance network offering 24-hour access to professional health and well-being support. - Career Growth Opportunities
ICON prioritizes internal mobility, offering opportunities to develop skills and move into leadership or specialist roles over time. - Inclusive and Respectful Culture
The company is deeply committed to creating an inclusive work environment where diversity is celebrated and everyone is treated with respect and fairness. - ICON plc Hiring Clinical Data Coordinator I
Application Process
Interested candidates who meet the eligibility criteria can apply for the Clinical Data Coordinator I (CDC I) position through the official ICON careers website. The online application form allows you to upload your resume, fill in required details, and track your application status. ICON plc Hiring Clinical Data Coordinator I
- Application Deadline: October 31, 2025
- Apply Mode: Online
- Official Website: https://careers.iconplc.com
Candidates currently employed at ICON should apply through the internal employee portal. ICON plc Hiring Clinical Data Coordinator I
Equal Opportunity and Accessibility
ICON plc is committed to providing a workplace that values diversity and ensures equal employment opportunities for all individuals. The company prohibits discrimination based on race, color, religion, gender, age, sexual orientation, national origin, disability, or veteran status.
If you have a medical condition or disability that requires special accommodation during the application or interview process, you are encouraged to contact ICON’s recruitment support team for assistance.
Important Dates
| Event | Date |
|---|---|
| Application Start Date | October 29, 2025 |
| Application End Date | October 31, 2025 |
| Mode of Application | Online Only |
| Job Type | Full Time |
| Locations | Bangalore, Trivandrum, Chennai |
Conclusion
The Clinical Data Coordinator I position at ICON plc offers a promising opportunity for professionals in clinical data management to advance their careers with a global leader in healthcare research. With its strong emphasis on innovation, data integrity, and teamwork, ICON provides an empowering work environment where you can contribute to meaningful projects and global health solutions. ICON plc Hiring Clinical Data Coordinator I
If you are passionate about data accuracy, clinical research, and contributing to medical advancement, this role could be the right step for you. Apply before October 31, 2025, to be part of ICON’s mission to shape the future of clinical development. ICON plc Hiring Clinical Data Coordinator I
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.
for delivering Latest Genuine Pharma Job Updates.

