Fortrea Hiring Freshers Experienced for Safety Science Coordinator in Pune for pharmacovigilance and clinical safety professionals. Apply before 11 December 2025.
Company Overview
Fortrea is a globally recognized Clinical Research Organization (CRO) delivering innovative solutions across clinical development, post-marketing safety, and regulatory services. With a strong presence in India and operations across multiple continents, Fortrea plays a critical role in advancing global healthcare through high-quality safety monitoring and regulatory compliance. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
The company is widely respected for its integrity, compliance-driven work culture, technology-backed safety systems, and commitment to professional growth for freshers as well as experienced professionals. Fortrea supports pharmaceutical, biotechnology, and medical device companies by offering end-to-end safety surveillance solutions.
Fortrea is now actively hiring for the position of Safety Science Coordinator I for its Pune location. This is a full-time role suitable for Pharmacy, Life Sciences, Nursing, and Medical Science graduates who want to build a strong career in Pharmacovigilance and Clinical Safety. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Key Highlight Box
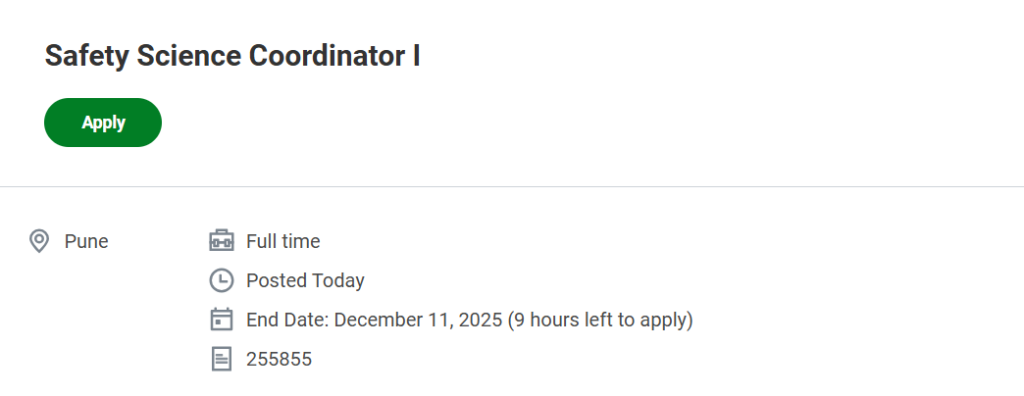
Job Title: Safety Science Coordinator I
Company: Fortrea
Location: Pune, Maharashtra, India
Job Type: Full Time
Job Category: Pharmacovigilance / Clinical Safety
Experience Required: Fresher to 2 Years
Education: BSc, MSc, BPharm, MPharm, PharmD, Nursing, Life Sciences
Application Deadline: 11 December 2025
Job ID: 255855
Shift: Office / Remote Based on Project
Industry: CRO / Pharmaceutical / Clinical Research
Job Overview
The Safety Science Coordinator I will assist with the complete lifecycle of Clinical Safety and Product Safety Surveillance operations. This role primarily focuses on the processing, tracking, documentation, and submission of adverse event reports arising from clinical trials as well as post-marketing safety data. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
The position involves working on Adverse Event (AE) and Serious Adverse Event (SAE) case processing, compliance with global regulatory timelines, MedDRA coding, narrative writing, and coordination of safety submissions to regulatory agencies, ethics committees, investigators, and sponsors.
This role ensures that all safety data is handled with the highest standards of quality, accuracy, confidentiality, and compliance with international regulatory laws. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Detailed Responsibilities
The selected candidate will be responsible for assisting and supporting global safety operations through the following activities: Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Adverse Event Processing
You will be involved in the processing of Expedited Safety Reports (ESRs) and Periodic Safety Reports (PSRs). All incoming AE and SAE reports must be logged into the safety tracking system and routed through the centralized project mailbox within strict timelines. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Safety Data Entry and Documentation
You will enter all safety data accurately into adverse event tracking systems. This includes maintaining project files, central documentation files, and ensuring all safety records remain audit-ready.
Patient Narrative Writing
You will write precise patient narratives by interpreting clinical data and reported events. This requires strong medical understanding and attention to clinical accuracy. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
MedDRA Coding
You will code adverse events accurately using the Medical Dictionary for Regulatory Activities (MedDRA). Proper term selection is crucial for downstream regulatory reporting and signal detection. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Listedness Assessment
For marketed products, you will assist with listedness evaluation by comparing reported reactions against approved product labels. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Query Generation
You will generate medical and data queries to collect missing, incomplete, or unclear safety information in consultation with medical reviewers.
Regulatory Submissions
You will support the submission of expedited SAE reports to clients, ethics committees, regulatory authorities, investigators, partners, and third-party vendors within globally specified timelines.
Quality Review and Reconciliation
You will assist with peer review and quality checks of processed safety cases. You will also support periodic database reconciliations between clinical and safety systems.
Regulatory File Maintenance
You will help maintain country-specific adverse event reporting requirements and regulatory submission logs across global markets.
Quality Management System Compliance
You will work strictly within the Quality Management System framework, including Standard Operating Procedures (SOPs), Work Instructions (WIs), and project-specific safety plans.
Safety File Archiving
You will help prepare and coordinate safety study files for secure archiving after project completion.
Meetings and Coordination
You will arrange and schedule internal and external teleconferences, safety review meetings, and compliance discussions.
Training and Mentorship
You will assist in training junior PSS Assistants and peers, supporting their daily operational tasks.
Cross-Functional Collaboration
You will maintain strong professional relationships across clinical, regulatory, data management, and medical writing teams.
Administrative Support
You will provide administrative and operational support to Pharmacovigilance and Product Safety teams.
Legal and Regulatory Compliance
The Safety Science Coordinator I must strictly adhere to: Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Health and Safety at Work Act 1974
COSHH Regulations 1989
European Commission Directives
Company Health and Safety Manual
Global Pharmacovigilance Regulatory Guidelines
Ensuring full compliance is a core responsibility of this role.
Educational Qualification Criteria
Candidates must meet at least one of the following:
Non-Degree professionals with 1–2 years of Safety or relevant experience
Associate Degree holders with 6 months to 1 year of Safety or relevant experience
BSc / BA graduates with 0–1 year of Safety or relevant experience
MSc / MA postgraduates with 0–6 months of Safety or relevant experience
PharmD graduates with 0–6 months of Safety or relevant experience
For PharmD candidates, a one-year residency or fellowship is considered valid experience. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Preferred academic disciplines include:
Biological Sciences
Pharmacy
Nursing
Medical Sciences
Life Sciences
Safety Experience Definition
Recognized safety experience includes:
Processing AE and SAE cases
Writing patient narratives
MedDRA coding
Working in safety databases
Regulatory safety submissions
Relevant Experience Considered
Even if formal safety experience is limited, experience from the following domains is also accepted: Fortrea Hiring Freshers Experienced for Safety Science Coordinator
Medical Affairs
Clinical Data Management
Clinical Monitoring
Regulatory Affairs
Quality Assurance
CRO Operations
Fortrea also considers relevant and equivalent experience in place of strict educational criteria.
Language Requirements
Speaking: English and local language
Reading/Writing: English and local language
Strong communication skills are mandatory for this role.
Essential Skills and Competencies
Strong attention to detail
Excellent time management and task prioritization
Ability to handle multiple projects simultaneously
Clear written and verbal communication
Logical thinking and high accuracy in documentation
Numerical proof-reading ability
Good keyboard skills
Working knowledge of MS Office and Windows
Ability to operate standard office equipment
Strong team player with peer support attitude
Work Environment
Office-based or Remote depending upon project allocation
Professional clinical research work environment
Global interaction with international safety teams
Career Growth and Benefits
This role provides long-term career growth into:
Drug Safety Associate
Senior Safety Specialist
Safety Scientist
Pharmacovigilance Project Lead
Global Safety Operations Manager
Fortrea offers structured training, global exposure, internal promotions, and continuous professional development in the high-demand pharmacovigilance domain.
Why You Should Apply
This opportunity is ideal for:
Pharmacy freshers entering Pharmacovigilance
Life Science graduates seeking CRO careers
Clinical research professionals switching into Drug Safety
Candidates seeking global regulatory exposure
Those who want stable growth in a compliance-driven industry
Job Location
This position is based in Pune, one of India’s largest hubs for clinical research, CRO operations, and pharmaceutical safety services.
Important Application Dates
Job Posted: Today
Last Date to Apply: 11 December 2025
Application Window Remaining: Limited Time
Candidates are strongly advised to apply immediately due to limited vacancies and high competition. Fortrea Hiring Freshers Experienced for Safety Science Coordinator
How to Apply
Interested and eligible candidates can apply directly through the official Fortrea careers portal by searching for the job requisition ID 255855.
Follow these steps carefully: Fortrea Hiring Freshers Experienced for Safety Science Coordinator
- Visit the official Fortrea career website
- Search for “Safety Science Coordinator I”
- Select the Pune location
- Use requisition ID 255855 to find the exact job
- Click on Apply
- Create or log in to your profile
- Upload your updated resume
- Fill in educational and experience details
- Submit your application before 11 December 2025
Ensure your resume highlights:
Pharmacovigilance exposure
AE/SAE knowledge
Clinical research experience
MedDRA familiarity
Regulatory reporting awareness
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



