Finecure Pharma Hiring for Production QC Jobs professionals via walk-in interview for experienced pharma candidates in Ahmedabad.
Introduction
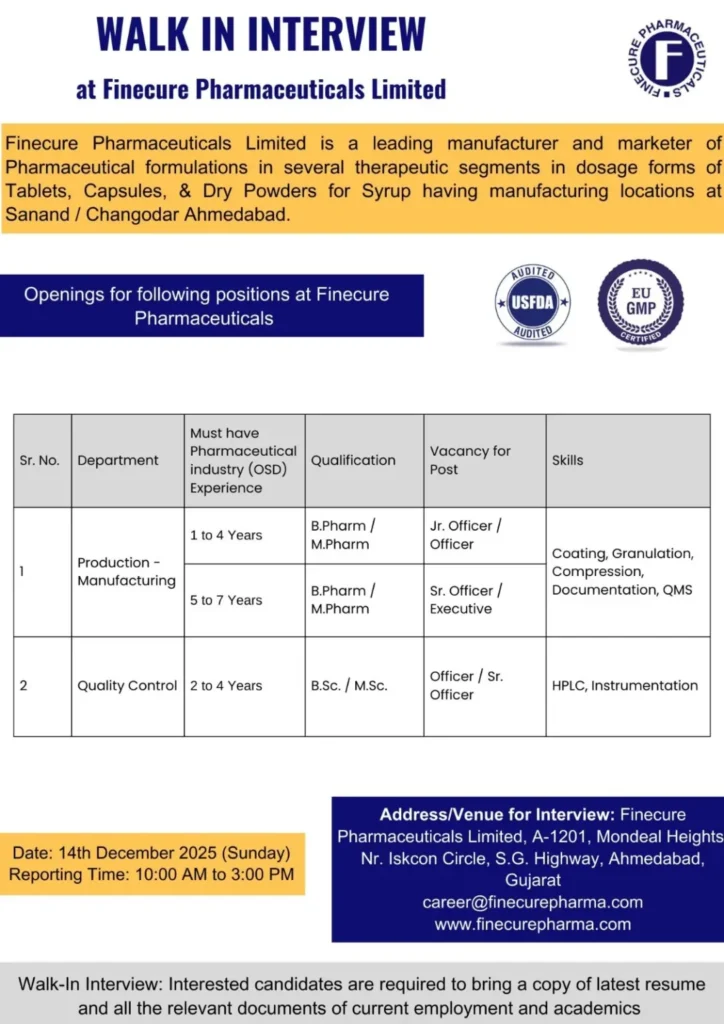
Finecure Pharmaceuticals Limited, a well-established pharmaceutical formulations manufacturer, has announced a walk-in interview drive for experienced professionals in the Production-Manufacturing and Quality Control (QC) departments. This recruitment initiative is aimed at strengthening the company’s operations at its Ahmedabad location with skilled candidates possessing hands-on experience in Oral Solid Dosage (OSD) formulations. Finecure Pharma Hiring for Production QC Jobs
This opportunity is ideal for B.Pharm, M.Pharm, B.Sc, and M.Sc graduates who are actively seeking pharma jobs in Ahmedabad within a regulated, quality-driven manufacturing environment. Candidates with prior exposure to tablets, capsules, and dry syrup formulations and a strong understanding of GMP and regulatory standards are strongly encouraged to attend.
The walk-in interview is scheduled for Sunday, 14th December 2025, and will be conducted directly at Finecure Pharmaceuticals’ corporate location in Ahmedabad. Finecure Pharma Hiring for Production QC Jobs
About Finecure Pharmaceuticals Limited
Finecure Pharmaceuticals Limited is a US-FDA and EU-GMP accredited pharmaceutical company engaged in the manufacturing of high-quality finished dosage formulations. The organization specializes in Oral Solid Dosage forms, including tablets, capsules, and dry powders for syrups, catering to both domestic and international markets. Finecure Pharma Hiring for Production QC Jobs
With state-of-the-art manufacturing facilities, validated processes, and robust quality systems, Finecure Pharmaceuticals maintains strict compliance with global regulatory guidelines. The company emphasizes product quality, process efficiency, regulatory compliance, and continuous improvement, making it a preferred workplace for pharmaceutical professionals seeking long-term career growth.
Employees at Finecure gain exposure to regulated manufacturing practices, analytical techniques, and documentation systems aligned with international standards. Finecure Pharma Hiring for Production QC Jobs
Key Highlights
Company Name: Finecure Pharmaceuticals Limited
Industry: Pharmaceutical Formulations (OSD)
Job Type: Full-Time, Permanent
Departments: Production-Manufacturing, Quality Control
Job Location: Ahmedabad, Gujarat
Experience Required: 1 to 7 Years
Qualification: B.Pharm, M.Pharm, B.Sc, M.Sc
Interview Type: Walk-In Interview
Interview Date: 14 December 2025 (Sunday)
Interview Time: 10:00 AM to 3:00 PM
Regulatory Exposure: US-FDA, EU-GMP
Salary Range: ₹3.5 LPA to ₹8 LPA (based on role and experience)
Walk-In Interview Details
Date: Sunday, 14 December 2025
Time: 10:00 AM to 3:00 PM
Venue:
Finecure Pharmaceuticals Limited
A-1201, Mondeal Heights,
Near Iskcon Circle,
S. G. Highway,
Ahmedabad, Gujarat – 380015
Contact Email: career@finecurepharma.com
Official Website: www.finecurepharma.com
Candidates are advised to report on time and carry all relevant documents for a smooth interview process. Finecure Pharma Hiring for Production QC Jobs
Available Positions and Eligibility Criteria
Finecure Pharmaceuticals is hiring experienced professionals for multiple roles across Production and Quality Control functions. Prior experience in pharmaceutical OSD manufacturing is mandatory. Finecure Pharma Hiring for Production QC Jobs
Production – Manufacturing Department
1. Junior Officer / Officer
Experience Required: 1 to 4 years
Qualification: B.Pharm or M.Pharm
Number of Vacancies: 1
Key Skills Required:
- Granulation (Wet/Dry)
- Compression operations
- Coating processes
- Manufacturing documentation
- QMS and GMP compliance
2. Senior Officer / Executive
Experience Required: 5 to 7 years
Qualification: B.Pharm or M.Pharm
Number of Vacancies: 2
Key Skills Required:
- Hands-on experience in OSD manufacturing
- Expertise in granulation, compression, and coating
- Batch manufacturing and packaging records
- Change control and deviation handling
- Strong knowledge of QMS and regulatory requirements
Quality Control Department
Officer / Senior Officer – Quality Control
Experience Required: 2 to 4 years
Qualification: B.Sc or M.Sc (Chemistry or relevant discipline)
Key Skills Required:
- HPLC operation and analysis
- Analytical instrumentation handling
- Method execution and documentation
- GMP and laboratory compliance
Roles and Responsibilities
Production – Manufacturing Roles
- Operate and monitor OSD manufacturing processes such as granulation, compression, and coating
- Ensure strict adherence to Good Manufacturing Practices (GMP) and safety guidelines
- Perform in-process checks and ensure batch consistency
- Handle batch manufacturing records, equipment logs, and SOP documentation
- Participate in QMS activities including deviations, CAPA, and change controls
- Troubleshoot manufacturing issues and support continuous process improvement
- Maintain cleanliness, equipment readiness, and compliance on the shop floor
Quality Control Roles
- Perform routine and non-routine analytical testing using HPLC and other instruments
- Conduct sampling and analysis of raw materials, in-process samples, and finished products
- Prepare, review, and maintain analytical reports and laboratory documentation
- Ensure compliance with GMP, GLP, and regulatory requirements
- Investigate out-of-specification (OOS) and out-of-trend (OOT) results
- Support stability studies and method verification activities
- Coordinate with production and QA teams to ensure timely product release
Required Qualifications and Skills
- Relevant educational qualification in Pharmacy or Science
- Mandatory experience in pharmaceutical OSD manufacturing or QC laboratory
- Strong understanding of GMP, regulatory guidelines, and quality systems
- Good documentation practices and attention to detail
- Ability to work in shifts and under production timelines
- Team-oriented mindset with effective communication skills
- Willingness to learn and adapt in a regulated manufacturing environment
Salary and Benefits
Finecure Pharmaceuticals offers competitive and performance-driven compensation based on candidate experience and role suitability. Finecure Pharma Hiring for Production QC Jobs
Indicative Salary Structure:
- Junior roles (1–4 years): ₹3.5 – ₹5.5 LPA
- Senior roles (5–7 years): ₹6 – ₹8 LPA
Benefits Include:
- Provident Fund (PF)
- Health insurance coverage
- Gratuity benefits
- Performance-based incentives
- Career growth and skill development opportunities
- Exposure to US-FDA and EU-GMP regulated operations
Final salary details will be discussed during the interview process. Finecure Pharma Hiring for Production QC Jobs
Why Choose Finecure Pharmaceuticals?
Finecure Pharmaceuticals provides a stable and growth-oriented work environment for pharmaceutical professionals. Employees benefit from structured systems, regulatory exposure, and hands-on operational roles that enhance both technical and professional competencies. Finecure Pharma Hiring for Production QC Jobs
For candidates seeking pharma manufacturing or QC jobs in Ahmedabad, this walk-in interview presents a valuable opportunity to join a reputable organization with global quality standards. Finecure Pharma Hiring for Production QC Jobs
How to Apply
Interested and eligible candidates can apply through either of the following methods:
- Attend the Walk-In Interview
Visit the interview venue on 14 December 2025 with:- Updated resume
- Educational certificates
- Experience letters
- Current salary details
- Email Your Resume
Send your updated CV to career@finecurepharma.com
Mention the department and position in the subject line of the email.
Candidates are advised to prepare well for technical discussions and bring all required documents for on-the-spot evaluation. Finecure Pharma Hiring for Production QC Jobs
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



