CIRO Pharma Hiring for Quality Control Roles professionals in Hyderabad through walk-in interview for RM, GLP, instrumentation and stability roles.
About the Company
CIRO Pharma Private Limited is a quality-driven pharmaceutical organization committed to excellence in formulation development, analytical testing, and regulatory compliance. The company operates with a strong focus on Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and data integrity, ensuring that all products meet stringent national and international regulatory standards. CIRO Pharma Hiring for Quality Control Roles
With a modern manufacturing and quality control facility located in Hyderabad, CIRO Pharma supports regulated and semi-regulated markets through robust quality systems, validated analytical methods, and a compliance-oriented work culture. The organization emphasizes continuous improvement, employee skill development, and adherence to global quality expectations.
To strengthen its Quality Control department, CIRO Pharma Private Limited is conducting walk-in interviews for experienced professionals who can contribute to analytical operations, GLP compliance, instrumentation management, and stability studies in a regulated pharmaceutical environment. CIRO Pharma Hiring for Quality Control Roles
Job Details
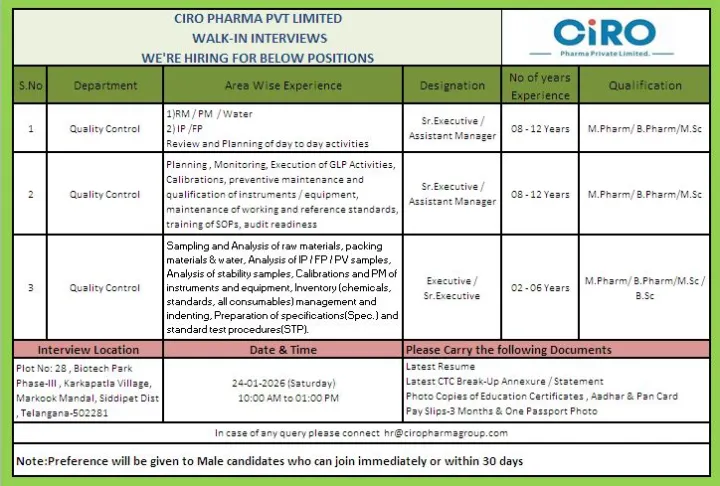
- Job Title: Quality Control Roles
- Company Name: CIRO Pharma Private Limited
- Department: Quality Control
- Job Location: Hyderabad, Telangana
- Employment Type: Full-Time
- Experience Required: 2 to 12 Years
- Educational Qualification: B.Pharm, M.Pharm, B.Sc, M.Sc
- Job Type: Walk-In Interview
- Interview Date: 24 January 2026
- Interview Time: 10:00 AM to 01:00 PM
- Job Status: Verified Job
Job Description
CIRO Pharma Private Limited is expanding its Quality Control operations and inviting experienced QC professionals to join its Hyderabad facility. The company is hiring across multiple QC functions, including Raw Material and Packing Material testing, water analysis, GLP and instrumentation management, finished product testing, and stability studies. CIRO Pharma Hiring for Quality Control Roles
Selected candidates will play a critical role in ensuring analytical accuracy, compliance with regulatory guidelines, and smooth day-to-day laboratory operations. These positions require hands-on experience in pharmaceutical quality control laboratories operating under GMP and GLP systems. CIRO Pharma Hiring for Quality Control Roles
The roles are best suited for candidates who have strong analytical skills, sound documentation practices, and the ability to work in audit-ready environments. CIRO Pharma offers a stable and growth-oriented platform for professionals seeking long-term careers in pharmaceutical quality control. CIRO Pharma Hiring for Quality Control Roles
Skills and Qualifications
Educational Qualifications
- B.Pharm or M.Pharm
- B.Sc or M.Sc in Chemistry, Pharmaceutical Sciences, or related disciplines
Experience Requirements
- Senior Executive / Assistant Manager: 8 to 12 years
- Executive / Senior Executive: 2 to 6 years
Core Skills
- Strong understanding of GMP and GLP requirements
- Experience in analytical testing and documentation
- Knowledge of stability studies and validation activities
- Familiarity with regulatory audits and inspections
- Ability to manage multiple QC activities and timelines
Key Responsibilities
Quality Control – RM / PM / Water
Designation: Senior Executive / Assistant Manager
Experience: 8–12 Years
- Perform analysis of raw materials, packing materials, and water samples
- Review In-Process (IP) and Finished Product (FP) analytical results
- Plan and manage daily QC laboratory activities
- Ensure timely completion of testing and accurate documentation
- Maintain compliance with regulatory and internal quality standards
- Support audit activities and regulatory inspections
CIRO Pharma Hiring for Quality Control Roles
Quality Control – GLP & Instrumentation
Designation: Senior Executive / Assistant Manager
Experience: 8–12 Years
- Plan, monitor, and implement GLP systems within the QC laboratory
- Manage calibration, preventive maintenance, and qualification of instruments
- Prepare, review, and maintain SOPs and GLP documentation
- Ensure audit readiness and regulatory compliance
- Conduct training for QC team members on GLP practices
- Manage working standards and reference standards
CIRO Pharma Hiring for Quality Control Roles
Quality Control – Finished Products & Stability
Designation: Executive / Senior Executive
Experience: 2–6 Years
- Perform sampling and analysis of raw materials, packing materials, and water
- Conduct In-Process (IP), Finished Product (FP), and Process Validation (PV) testing
- Support stability studies as per approved protocols
- Handle calibration activities and laboratory equipment usage
- Manage laboratory chemicals, consumables, and standards
- Prepare Specifications (STP) and Standard Operating Procedures (SOPs)
CIRO Pharma Hiring for Quality Control Roles
Benefits and Perks
CIRO Pharma Private Limited offers competitive compensation packages aligned with industry standards and individual experience levels. CIRO Pharma Hiring for Quality Control Roles
Salary Range (Indicative)
- Executive / Senior Executive: ₹4.5 – ₹7.5 LPA
- Senior Executive / Assistant Manager: ₹8.0 – ₹12.0 LPA
Additional Benefits
- Performance-based incentives
- Health insurance coverage
- Stable work environment in a GMP-compliant facility
- Professional skill development and training
- Exposure to regulatory audits and quality systems
Why You Should Join CIRO Pharma
CIRO Pharma provides an excellent opportunity for Quality Control professionals to work in a compliance-focused and growth-oriented organization. The company emphasizes analytical accuracy, strong quality systems, and continuous learning, making it an ideal workplace for professionals seeking long-term career development.
Employees gain hands-on exposure to GLP implementation, instrumentation management, stability studies, and regulatory inspection readiness. With a structured quality culture and supportive leadership, CIRO Pharma enables professionals to enhance both technical expertise and regulatory understanding. CIRO Pharma Hiring for Quality Control Roles
FAQs
Who can attend this walk-in interview?
Experienced Quality Control professionals with 2 to 12 years of relevant experience can attend.
Is GLP experience mandatory?
GLP experience is mandatory for GLP and Instrumentation roles and preferred for senior QC positions.
Are freshers eligible?
No, these roles are intended for experienced candidates only.
Is immediate joining preferred?
Yes, preference will be given to candidates who can join immediately or within 30 days.
Does CIRO Pharma charge any recruitment fees?
No. CIRO Pharma does not charge any fees for recruitment.
How to Apply
Interested candidates should attend the walk-in interview directly with all required documents.
Walk-In Interview Details:
Date: 24 January 2026 (Saturday)
Time: 10:00 AM to 01:00 PM
Interview Venue:
CIRO Pharma Private Limited
Plot No. 28, Biotech Park, Phase-III
Karkapatla Village, Markook Mandal
Siddipet District, Telangana – 502281
Documents Required:
- Updated resume
- Latest CTC break-up / annexure / statement
- Photocopies of educational certificates
- Aadhaar card and PAN card copies
- Last three months’ payslips
- One passport-size photograph
For any pre-interview queries, candidates may contact the HR team via email at hr@ciropharmagroup.com.
This walk-in interview presents a strong opportunity to join CIRO Pharma Private Limited and build a rewarding career in pharmaceutical Quality Control within a GMP- and GLP-compliant environment. CIRO Pharma Hiring for Quality Control Roles
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



