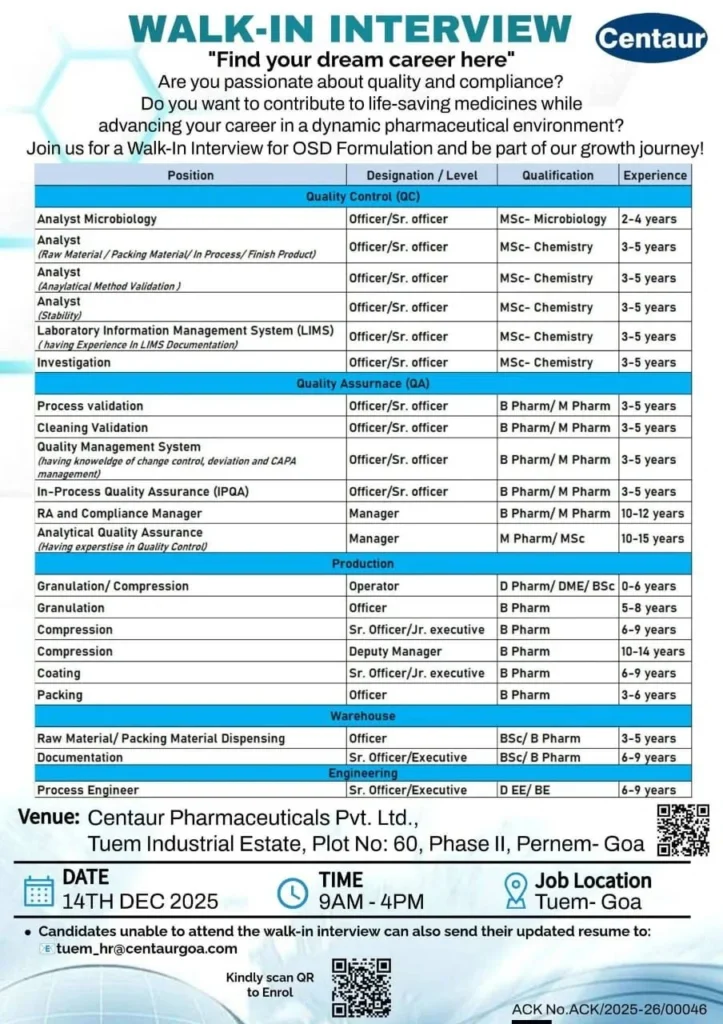
Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering roles through Goa walk-in interview December 2025.
If you are a qualified candidate from the pharmaceutical, science, engineering, or technical background searching for a stable and high-growth career opportunity, then this recruitment drive is extremely valuable for you. Centaur Pharmaceuticals Pvt. Ltd., one of India’s fastest-growing pharmaceutical companies, has officially announced a mega Walk-In Interview on 14 December 2025 (Sunday) at its modern Oral Solid Dosage (OSD) formulation facility located in Tuem Industrial Estate, Goa. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
This hiring drive is open for a wide range of positions across Quality Control (QC), Quality Assurance (QA), Production, Warehouse, and Engineering departments. More than 20+ job openings are available for professionals with 2 to 15 years of experience, making this walk-in suitable for junior, mid-level, and senior pharmaceutical professionals. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
Centaur Pharmaceuticals is widely known for its strong product quality, regulatory compliance, innovation-driven manufacturing, and employee-centric work culture. The company operates WHO-GMP, USFDA, and EU-approved manufacturing facilities, producing high-quality formulations for domestic and international markets. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
About Centaur Pharmaceuticals
Centaur Pharmaceuticals Pvt. Ltd. is a leading Indian pharmaceutical company engaged in the development, manufacturing, and marketing of high-quality generic formulations. The company has built its reputation on trust, compliance, product excellence, and customer satisfaction. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
With a strong footprint in domestic markets and an expanding global presence, Centaur operates advanced formulation manufacturing facilities that comply with global regulatory standards such as WHO-GMP, USFDA, MHRA, and EU certifications. The company’s strength lies in: Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
- Oral solid dosage formulations
- Advanced quality systems
- Research-driven product development
- Regulatory excellence
- Skilled manufacturing workforce
The Tuem, Goa plant is one of Centaur’s most advanced manufacturing sites, delivering pharmaceutical products to global regulated markets.
Walk-In Interview Event Details – Goa
Centaur Pharmaceuticals has scheduled this recruitment drive with the following official details: Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
- Interview Date: 14 December 2025 (Sunday)
- Reporting Time: 09:00 AM to 04:00 PM
- Venue:
Centaur Pharmaceuticals Pvt. Ltd.
Plot No. 60, Phase II,
Tuem Industrial Estate,
Pernem, Goa - Alternate Application Mode:
Candidates unable to attend can email their updated resume to
tuem.hr@centaurgoa.com
This walk-in is targeted at experienced professionals who are ready to work in a regulated pharmaceutical manufacturing environment. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
Key Highlight Box
Company Name: Centaur Pharmaceuticals Pvt. Ltd.
Job Location: Tuem Industrial Estate, Pernem, Goa
Interview Type: Walk-In Interview
Interview Date: 14 December 2025
Departments Hiring: QC, QA, Production, Warehouse, Engineering
Experience Required: 2 to 15 Years
Qualification Required: B.Sc, M.Sc, B.Pharm, M.Pharm, D.Pharm, ITI, B.E, B.Tech
Industry: Pharmaceutical Formulations
Plant Type: WHO-GMP, USFDA & EU Approved
Salary Range: ₹3 LPA to ₹25 LPA
Employment Type: Full-Time
Hiring Level: Junior to Senior Management
Educational Qualification Criteria
Candidates must possess one of the following qualifications depending on the department:
- B.Sc
- M.Sc
- B.Pharm
- M.Pharm
- D.Pharm
- ITI (Technical Trades)
- B.E
- B.Tech (Mechanical / Chemical)
Only candidates with relevant pharmaceutical, engineering, or quality experience should attend this walk-in interview. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
Department-Wise Open Positions & Eligibility
Quality Control (QC) Department Vacancies
These roles focus on raw material testing, in-process checks, finished product analysis, documentation, stability testing, and microbiology. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
- Microbiology Analyst
Qualification: M.Sc Microbiology
Experience: 2 to 4 years - Analyst – Raw Material / Packing Material / In-Process / Finished Product
Qualification: M.Sc Chemistry
Experience: 3 to 5 years - Analytical Method Validation / Stability Testing
Qualification: M.Sc Chemistry
Experience: 3 to 5 years - LIMS Documentation Executive
Qualification: M.Sc Chemistry
Experience: 3 to 5 years - Investigation Officer
Qualification: M.Sc Chemistry
Experience: 3 to 5 years
Quality Assurance (QA) Department Vacancies
QA roles are critical for ensuring regulatory compliance, audit readiness, documentation systems, and quality management. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
- Process Validation / Cleaning Validation Executive
Qualification: B.Pharm / M.Pharm
Experience: 3 to 5 years - Quality Management System (Change Control, Deviation, CAPA)
Qualification: B.Pharm / M.Pharm
Experience: 3 to 5 years - In-Process Quality Assurance (IPQA)
Qualification: B.Pharm / M.Pharm
Experience: 3 to 5 years - Regulatory Affairs & Compliance Manager
Qualification: B.Pharm / M.Pharm
Experience: 10 to 12 years - Analytical Quality Assurance Head
Qualification: M.Pharm / M.Sc
Experience: 10 to 15 years
Production Department Vacancies
Production professionals will be responsible for solid dosage manufacturing, equipment handling, batch execution, and productivity improvement. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
- Granulation / Compression / Coating / Packing
Level: Operator to Senior Officer
Qualification: D.Pharm / B.Pharm / ITI - Granulation Officer
Qualification: B.Pharm
Experience: 5 to 8 years - Compression Deputy Manager
Qualification: B.Pharm
Experience: 10 to 14 years
Warehouse Department Vacancies
Warehouse roles manage dispensing, documentation, and material tracking.
- Raw Material / Packing Material Dispensing Executive
Qualification: B.Sc / B.Pharm
Experience: 3 to 5 years - Warehouse Documentation Executive
Qualification: B.Sc / B.Pharm
Experience: 6 to 9 years
Engineering Department Vacancies
Engineering professionals ensure smooth plant operations and equipment reliability.
- Process Engineer
Qualification: B.E / B.Tech (Mechanical / Chemical)
Experience: 6 to 9 years
Salary Structure & Compensation (Expected)
Centaur Pharmaceuticals is known for offering competitive salary packages aligned with industry standards.
- Freshers & Junior Professionals: ₹3.0 – ₹5.5 LPA
- Mid-Level Professionals (3–8 Years): ₹6.0 – ₹12 LPA
- Senior & Managerial Roles: ₹15 – ₹25 LPA
Final salary will depend on:
- Current CTC
- Relevant experience
- Skill level
- Interview performance
- Department requirements
Additional Benefits & Employee Facilities
Selected candidates will receive attractive benefits including:
- Free transportation facility
- Subsidized canteen food
- Group mediclaim insurance
- Personal accident insurance
- Performance-linked incentives
- Regulated and safe working environment
- Exposure to USFDA and EU regulatory audits
- Excellent career growth opportunities
Work Culture & Professional Growth
Centaur Pharmaceuticals promotes a performance-oriented and ethical culture. Employees benefit from continuous learning, exposure to global regulatory practices, leadership mentoring, and operational excellence programs. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
Employees working at regulated plants gain valuable experience that strengthens their professional profile across the pharmaceutical industry. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
Selection Process
Candidates attending the walk-in interview will go through the following stages:
- Registration & Resume Screening
- Technical Interview
- HR Interaction
- Salary Discussion
- Document Verification
- Final Selection
Selected candidates will receive appointment confirmation after interview clearance.
Documents Required for Interview
Candidates must bring the following documents:
- Updated resume
- Two passport-size photographs
- Last three months’ salary slips
- All educational certificates
- Experience certificates
- Government ID proof
Incomplete documentation may lead to rejection.
Who Should Attend This Walk-In Interview
This recruitment drive is ideal for: Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
- QC and QA professionals with regulated plant experience
- Production officers and operators in OSD formulations
- Warehouse executives with dispensing experience
- Engineering professionals from pharma backgrounds
- Candidates seeking USFDA and EU exposure
- Professionals aiming for high-growth formulation careers
Important Instructions for Candidates
- Reach the venue before 09:00 AM
- Carry all original documents
- Dress formally
- Mention the exact position you are applying for
- Be prepared for technical discussion
- Follow safety and discipline guidelines at the plant
How to Apply
This recruitment is being conducted through direct walk-in interview only.
Eligible candidates must:
- Visit the interview venue on 14 December 2025
- Reach between 9:00 AM to 4:00 PM
- Carry updated resume, photographs, salary slips, and all educational certificates
- Attend the interview at Centaur Pharmaceuticals, Tuem Industrial Estate, Goa
For Candidates Unable to Attend Walk-In
You may email your updated resume with the subject line:
“Position Applied For_Walk-In Dec 2025”
to the official HR email address:
Only shortlisted candidates will be contacted via email or phone. Centaur Pharmaceuticals Hiring for QC QA Production Warehouse Engineering
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.


