Clinical Research Associate Job in New Delhi at George Institute. Apply for global clinical trials and impactful healthcare research opportunities.
Clinical research careers continue to grow in India, especially with global institutes expanding large-scale trials across multiple countries. The George Institute for Global Health is hiring Clinical Research Associates (CRA) in New Delhi for multi-country clinical research projects. This opportunity is ideal for candidates with experience in clinical trial coordination and monitoring who want to work on impactful global healthcare studies.
Professionals interested in contributing to large clinical trials focused on chronic diseases and global health outcomes will find this role highly meaningful and career-enhancing. Clinical Research Associate Job in New Delhi
Organization Overview
The George Institute for Global Health is an internationally recognized medical research organization working to improve health outcomes worldwide. With a presence in over 40 countries and a workforce of 700+ professionals, the institute focuses on solving major global health challenges through research and innovation. Clinical Research Associate Job in New Delhi
Its work spans cardiovascular diseases, kidney disorders, public health initiatives, and digital health innovations. The organization collaborates with leading universities and research bodies globally, aiming to improve healthcare access, especially in low- and middle-income regions.
The India entity operates through George Institute Services India Pvt. Ltd., which supports research, advisory services, and consulting initiatives across healthcare and life sciences. Clinical Research Associate Job in New Delhi
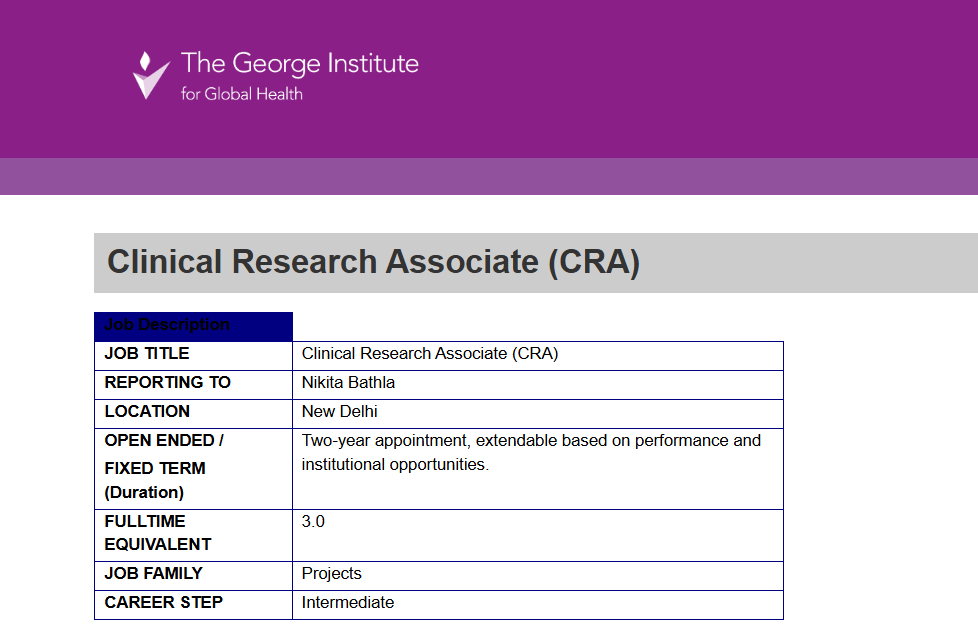
Job Location & Employment Type
- Position: Clinical Research Associate (CRA)
- Location: New Delhi, India
- Employment Type: Fixed-Term (Two-Year Contract, extendable)
- Department: Clinical Research Projects
- Reporting To: Senior Project Manager
Role Overview
The Clinical Research Associate will support large-scale clinical trials across multiple sites and countries. The role involves managing trial documentation, coordinating with regulatory authorities, and monitoring study sites throughout the trial lifecycle. Clinical Research Associate Job in New Delhi
CRAs will be involved in trial setup, monitoring visits, data verification, and site closure activities. This position offers exposure to international research standards and real-world clinical impact. Clinical Research Associate Job in New Delhi
Key Roles & Responsibilities
Clinical Trial Coordination
- Supporting development of trial-related documents such as protocols and patient materials
- Assisting in ethics committee and regulatory authority coordination
- Evaluating clinical trial sites for suitability
- Supporting investigator selection and site readiness
Site Setup & Initiation
- Ensuring availability of trial materials and investigational products
- Training site teams on trial protocols and procedures
- Supporting study initiation processes
Monitoring & Site Management
- Conducting regular monitoring visits across trial sites
- Verifying source data and case report forms (CRFs)
- Resolving operational issues during trials
- Preparing monitoring visit reports
Documentation & Compliance
- Maintaining trial master files and documentation
- Ensuring accurate data collection and reporting
- Archiving clinical trial documentation
- Preparing final reports for sponsors and authorities
Clinical Research Associate Job in New Delhi
Trial Closure Activities
- Ensuring accountability of investigational products
- Closing trial sites after completion
- Archiving study records as per compliance requirements
Team Collaboration
- Participating in team discussions and research initiatives
- Supporting process improvements and innovation projects
- Maintaining alignment with institutional values and ethics
Eligibility Criteria
Educational Qualification
Candidates must have a background in:
- Life Sciences
- Medical Sciences
- Nursing
- Clinical Research
Relevant academic qualifications in healthcare or biomedical sciences are preferred.
Experience Required
- Minimum 2 years of experience in clinical trial coordination or monitoring
- Experience working on clinical trials preferred
- Willingness to travel frequently for site visits
Skills Required
Candidates should demonstrate strong clinical research competencies and operational skills.
Technical Skills:
- Knowledge of clinical trial processes
- Understanding of regulatory and ethics requirements
- Familiarity with CRFs and source data verification
- Clinical documentation experience
Core Competencies:
- Strong analytical and problem-solving abilities
- Excellent communication and interpersonal skills
- Ability to work independently and within teams
- Strong organizational skills and attention to detail
- Adaptability to dynamic research environments
Behavioral Attributes:
- High ethical standards and integrity
- Commitment to quality and accuracy
- Flexibility and resilience under pressure
- Collaborative mindset
Salary & Benefits
Salary will be as per company norms and aligned with candidate experience and project requirements.
Working with a global research institute may offer:
- Exposure to international clinical trials
- Opportunities for learning and development
- Meaningful contribution to global health initiatives
- Collaborative research environment
- Career growth in academic and clinical research sectors
Selection Process
The hiring process may involve:
- Resume screening
- Technical evaluation
- Interview with research leadership
- Final selection and onboarding
Only shortlisted candidates will be contacted for further steps.
How to Apply
Interested candidates should apply via email by sharing:
- Updated resume
- Current and expected salary details
- Notice period information
Email Subject Format: Clinical Research Associate (CRA)
Application Email: hrindia@georgeinstitute.org.in
Ensure the subject line is mentioned correctly to avoid application rejection.
Important Dates
- Contract Duration: Two-year appointment (extendable)
- Candidates are encouraged to apply early as positions may close once filled.
Why Apply for This Clinical Research Job?
This opportunity offers strong career value for clinical research professionals looking to work in impactful global projects. Clinical Research Associate Job in New Delhi
Key advantages include:
- Work with an internationally reputed research institute
- Involvement in multi-country clinical trials
- Contribution to global health research
- Exposure to advanced clinical methodologies
- Strong learning opportunities in research environments
- Career growth in academic and global research sectors
This role is especially beneficial for CRAs aiming to transition into senior clinical research or project management roles. Clinical Research Associate Job in New Delhi
Important Note / Disclaimer
PharmaJobhub.in is an independent pharmaceutical job information platform designed to help job seekers discover verified opportunities. We are not affiliated with The George Institute or any hiring organization. Candidates should verify job details before applying. We do not charge any fee for job listings or applications. Clinical Research Associate Job in New Delhi
Final Call-to-Action
If you have experience in clinical trials and want to work on high-impact global research projects, this Clinical Research Associate opportunity in New Delhi is worth exploring. Prepare your updated resume and apply via email as soon as possible. Follow IndiaPharmaJobs.in for the latest clinical research, pharma, and healthcare job updates across India. Clinical Research Associate Job in New Delhi



