Zydus Lifesciences Hiring Vaccine Manufacturing Professionals Job
About the Company
Zydus Lifesciences Limited is a globally recognized pharmaceutical and life sciences organization with a strong presence across vaccines, biologics, generics, and innovative healthcare solutions. The company is known for its commitment to scientific excellence, quality manufacturing practices, and continuous investment in advanced technologies. Hiring Vaccine Manufacturing Professionals Job
With state-of-the-art facilities and a robust research-driven approach, Zydus Lifesciences plays a significant role in vaccine development and manufacturing for domestic and international markets. Its Vaccine Technology Centre in Ahmedabad is a key hub for large-scale vaccine manufacturing, cell culture operations, and bacterial vaccine production. Hiring Vaccine Manufacturing Professionals Job
Working at Zydus Lifesciences means being part of an organization that values innovation, compliance, patient safety, and professional growth. The company offers a collaborative environment where experienced professionals can expand their technical expertise while contributing to impactful healthcare solutions. Hiring Vaccine Manufacturing Professionals Job
Job Details
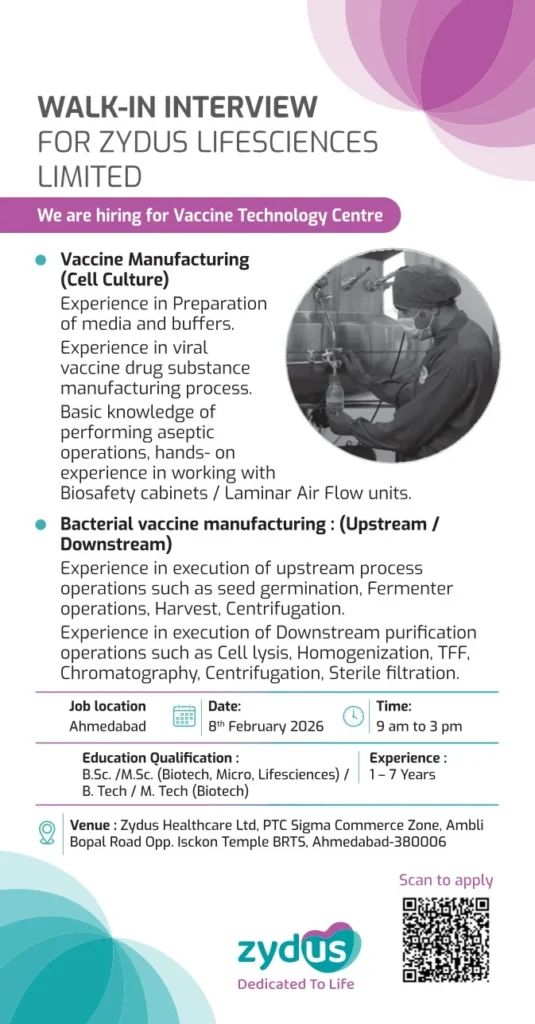
- Job Title: Vaccine Manufacturing Professionals
- Department: Vaccine Technology Centre
- Company: Zydus Lifesciences Limited
- Job Location: Ahmedabad, Gujarat, India
- Employment Type: Full-Time
- Hiring Mode: Walk-In Interview
- Experience Required: 1 to 7 Years
- Educational Qualification:
- B.Sc / M.Sc (Biotechnology, Microbiology, Life Sciences)
- B.Tech / M.Tech (Biotechnology)
- Industry: Pharmaceutical / Biotechnology / Vaccine Manufacturing
This opportunity is ideal for professionals searching for vaccine manufacturing jobs in Ahmedabad, biotech production roles, or careers in microbial and cell culture-based vaccine manufacturing. Hiring Vaccine Manufacturing Professionals Job
Job Description
Zydus Lifesciences is hiring experienced Vaccine Manufacturing professionals to support its expanding operations at the Vaccine Technology Centre. The selected candidates will be involved in end-to-end vaccine manufacturing activities, including viral vaccine drug substance production and bacterial vaccine upstream and downstream processes. Hiring Vaccine Manufacturing Professionals Job
The role requires hands-on experience in aseptic manufacturing environments, adherence to GMP standards, and execution of complex bioprocessing operations. Candidates will work closely with cross-functional teams to ensure safe, efficient, and compliant manufacturing of vaccine products.
This position is suited for professionals with a strong background in biotechnology, microbiology, or life sciences who are looking to advance their careers in large-scale vaccine manufacturing within a globally reputed pharmaceutical organization. Hiring Vaccine Manufacturing Professionals Job
Skills and Qualifications
Educational Qualifications
Candidates must possess one of the following qualifications:
- B.Tech or M.Tech in Biotechnology
- B.Sc or M.Sc in Biotechnology, Microbiology, or Life Sciences
Experience Requirements
- Minimum 1 year and up to 7 years of relevant experience in vaccine manufacturing, cell culture operations, or bacterial fermentation processes
Technical and Professional Skills
- Hands-on experience in vaccine drug substance manufacturing
- Knowledge of GMP and aseptic manufacturing practices
- Experience working in controlled cleanroom environments
- Understanding of upstream and downstream bioprocessing
- Ability to follow SOPs, batch manufacturing records, and safety protocols
- Strong documentation and compliance mindset
- Team-oriented approach with good communication skills
Hiring Vaccine Manufacturing Professionals Job
Key Responsibilities
Selected candidates will be responsible for the following activities within vaccine manufacturing operations: Hiring Vaccine Manufacturing Professionals Job
Viral Vaccine Manufacturing
- Preparation of media and buffers for vaccine manufacturing processes
- Execution of viral vaccine drug substance manufacturing steps
- Performing aseptic operations inside Biosafety Cabinets and Laminar Air Flow units
- Adherence to GMP and biosafety guidelines during manufacturing activities
Bacterial Vaccine Upstream Operations
- Seed germination and culture preparation
- Fermenter operations and monitoring of critical process parameters
- Harvesting and centrifugation of bacterial cultures
Bacterial Vaccine Downstream Operations
- Cell lysis and homogenization processes
- Tangential Flow Filtration (TFF)
- Chromatography operations
- Centrifugation and sterile filtration
- Ensuring product quality and yield during purification steps
General Responsibilities
- Accurate documentation of manufacturing activities
- Compliance with quality, safety, and regulatory requirements
- Coordination with quality assurance and support teams
- Participation in audits, investigations, and process improvements
Benefits and Perks
Zydus Lifesciences offers a comprehensive set of benefits to its vaccine manufacturing professionals, including: Hiring Vaccine Manufacturing Professionals Job
- Opportunity to work with a global pharmaceutical leader
- Exposure to advanced vaccine manufacturing technologies
- Competitive salary structure aligned with industry standards
- Strong learning and professional development opportunities
- Stable and growth-oriented work environment
- Experience with large-scale commercial vaccine production
- Long-term career progression in biologics and vaccines
Why You Should Join Zydus Lifesciences
Joining Zydus Lifesciences provides an opportunity to be part of an organization that directly contributes to public health through vaccine development and manufacturing. The Vaccine Technology Centre in Ahmedabad offers exposure to cutting-edge bioprocessing technologies and large-scale production systems.
For professionals with experience in cell culture, fermentation, or downstream processing, this role offers a platform to enhance technical capabilities while working on critical vaccine programs. Zydus Lifesciences emphasizes quality, innovation, and continuous improvement, making it an ideal workplace for experienced biotech and pharmaceutical professionals seeking meaningful and stable careers.
Walk-In Interview Details
Interested and eligible candidates are invited to attend the walk-in interview as per the details below: Hiring Vaccine Manufacturing Professionals Job
- Interview Date: 8 February 2026
- Interview Time: 9:00 AM to 3:00 PM
Interview Venue:
Zydus Healthcare Ltd
PTC Sigma Commerce Zone
Ambli Bopal Road, Opposite ISKCON Temple BRTS
Ahmedabad – 380006
Candidates are advised to carry updated resumes, educational certificates, experience documents, and identity proof while attending the interview.
Frequently Asked Questions (FAQs)
Is this opportunity open to freshers?
No, this role requires prior experience. Candidates must have 1 to 7 years of relevant vaccine manufacturing or bioprocessing experience.
Which departments are hiring under this drive?
The hiring is for the Vaccine Technology Centre, covering viral vaccine and bacterial vaccine manufacturing operations.
Is this a walk-in interview or online process?
This is a walk-in interview. Interested candidates must visit the venue on the scheduled date and time.
What kind of experience is preferred?
Experience in cell culture, viral vaccine drug substance manufacturing, bacterial fermentation, and downstream processing is preferred.
Where is the job location?
The job location is Ahmedabad, Gujarat, India.
How to Apply
This is a direct walk-in interview opportunity. Eligible candidates should attend the interview in person at the specified venue on 8 February 2026 between 9:00 AM and 3:00 PM with all required documents. No prior online application is mandatory for this hiring drive. Hiring Vaccine Manufacturing Professionals Job
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



