Finecure Pharmaceuticals Hiring for Production QA QC ADL Engineering Roles in Gujarat through walk-in interview.
Job Type: Full-Time
Industry: Pharmaceutical Manufacturing
Job Categories: Production, Quality Assurance, Quality Control, Analytical Development, Engineering
Experience Required: 1–7 Years
Location: Ahmedabad, Gujarat
Interview Type: Walk-In Interview
About the Company
Finecure Pharmaceuticals Limited is a well-established pharmaceutical formulations manufacturer based in Ahmedabad, Gujarat, with a strong focus on quality-driven and regulatory-compliant manufacturing. The company is USFDA and EU-GMP certified, reflecting its commitment to meeting international quality standards and supplying regulated markets.
Finecure Pharmaceuticals specializes in the manufacturing of tablets, capsules, and dry powder syrups within the Oral Solid Dosage (OSD) segment. The organization operates modern, well-equipped manufacturing facilities located at Sanand and Changodar (Ahmedabad), supported by advanced analytical laboratories and robust quality systems.
Over the years, Finecure has built a reputation for operational excellence, compliance integrity, and continuous improvement. The company places strong emphasis on employee development, technical training, and maintaining a collaborative work environment, making it an attractive employer for pharmaceutical professionals seeking stable and long-term career growth. Hiring for Production QA QC ADL Engineering Roles
Job Details
Finecure Pharmaceuticals Limited is conducting a walk-in interview to hire experienced professionals across multiple departments including Production, Quality Assurance, Quality Control, Analytical Development Laboratory (ADL), and Engineering. Hiring for Production QA QC ADL Engineering Roles
This hiring drive is aimed at strengthening its manufacturing and quality teams to support ongoing and future expansion plans.
Job Location
- Ahmedabad, Gujarat
- Manufacturing units located at Sanand and Changodar
Employment Type
- Permanent, Full-Time
Eligible Qualifications
- M.Pharm
- M.Sc
- B.Pharm
- B.Sc
- D.Pharm
- ITI / Diploma (for Engineering roles)
Job Description
Selected candidates will be responsible for performing departmental operations in compliance with Good Manufacturing Practices (GMP), USFDA, and EU-GMP regulatory standards. The roles require hands-on experience in pharmaceutical manufacturing, quality systems, analytical testing, or engineering maintenance, depending on the department.
Professionals joining Finecure will work in a regulated manufacturing environment where accuracy, documentation, and compliance are critical. The organization offers exposure to modern pharmaceutical processes, validated systems, and international audit standards. Hiring for Production QA QC ADL Engineering Roles
Skills / Qualifications
General Eligibility Criteria
- Relevant academic qualification as per the applied department
- Minimum 1 year to maximum 7 years of experience in pharmaceutical manufacturing or quality functions
- Prior experience in OSD formulation is strongly preferred
- Sound understanding of GMP, SOPs, and regulatory documentation
Hiring for Production QA QC ADL Engineering Roles
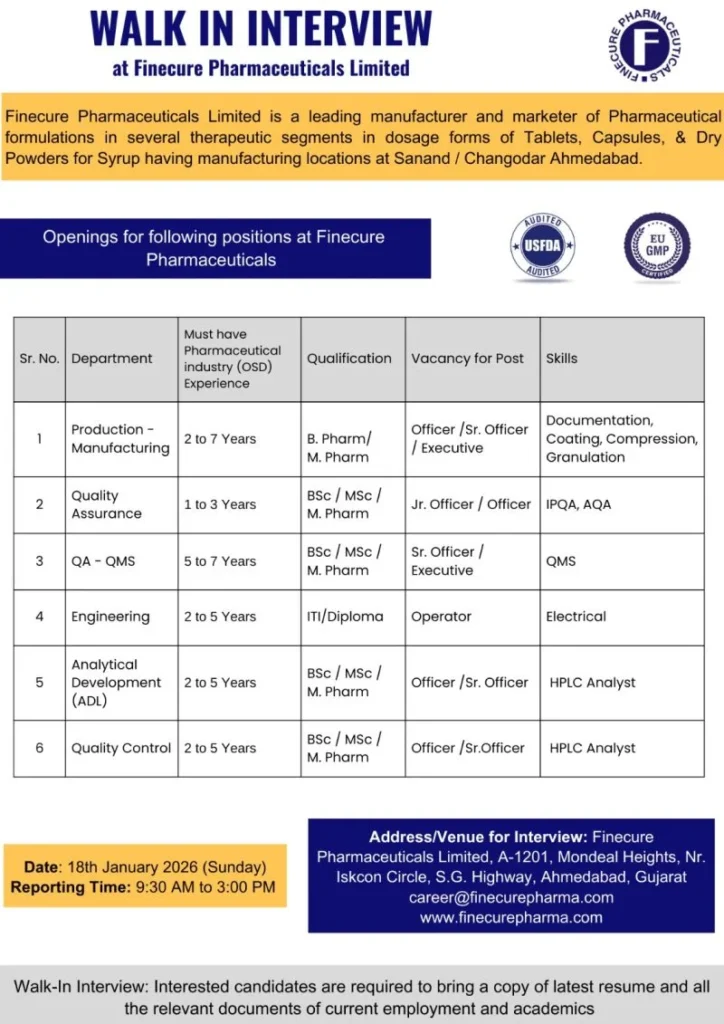
Key Open Positions and Department-Wise Requirements
Production Department – OSD Manufacturing
- Experience: 2 to 7 years
- Qualification: B.Pharm / M.Pharm
- Positions: Officer / Senior Officer / Executive
- Key Skills:
- Granulation, Compression, Coating
- Batch Manufacturing Records (BMR)
- Equipment operation and process control
- Documentation and cGMP compliance
Hiring for Production QA QC ADL Engineering Roles
Quality Assurance (IPQA / AQA)
- Experience: 1 to 3 years
- Qualification: B.Sc / M.Sc / M.Pharm
- Positions: Junior Officer / Officer
- Key Skills:
- In-Process Quality Assurance (IPQA)
- Analytical Quality Assurance (AQA)
- Line clearance and compliance monitoring
- Deviation handling and documentation
Hiring for Production QA QC ADL Engineering Roles
Quality Assurance – QMS
- Experience: 5 to 7 years
- Qualification: B.Sc / M.Sc / M.Pharm
- Positions: Senior Officer / Executive
- Key Skills:
- Quality Management System (QMS)
- Change control, deviation, CAPA
- Audit coordination and documentation
- Regulatory compliance and SOP management
Quality Control (QC)
- Experience: 2 to 5 years
- Qualification: B.Sc / M.Sc / M.Pharm
- Positions: Officer / Senior Officer
- Key Skills:
- HPLC analysis
- Raw material, in-process, and finished product testing
- Stability studies and documentation
- GMP and data integrity compliance
Hiring for Production QA QC ADL Engineering Roles
Analytical Development Laboratory (ADL)
- Experience: 2 to 5 years
- Qualification: B.Sc / M.Sc / M.Pharm
- Positions: Officer / Senior Officer
- Key Skills:
- HPLC method development and validation
- Analytical method transfer
- Regulatory documentation support
- Stability and troubleshooting activities
Engineering Department
- Experience: 2 to 5 years
- Qualification: ITI / Diploma
- Positions: Operator
- Key Skills:
- Electrical maintenance
- Equipment troubleshooting
- Preventive and breakdown maintenance
- Utility and plant support operations
Hiring for Production QA QC ADL Engineering Roles
Key Responsibilities
General Responsibilities Across Departments
- Execute daily operational activities as per SOPs and GMP guidelines
- Maintain accurate and timely documentation
- Ensure compliance with USFDA, EU-GMP, and internal quality standards
- Participate in audits, inspections, and regulatory interactions
- Follow safety, hygiene, and data integrity practices
Department-Specific Responsibilities
- Production: Operate and monitor OSD manufacturing processes including granulation, compression, and coating
- QA: Perform line clearance, IPQA checks, deviation investigations, and QMS documentation
- QC / ADL: Conduct analytical testing using HPLC and other instruments, review data, and support validations
- Engineering: Maintain electrical systems, equipment uptime, and plant utilities
Hiring for Production QA QC ADL Engineering Roles
Benefits / Perks
Finecure Pharmaceuticals offers competitive compensation packages aligned with industry benchmarks in Gujarat.
Salary Range
- Expected CTC: INR 3.0 Lakhs to 8.0 Lakhs per annum
- Salary varies based on role, experience, and position level
- Senior and specialized roles may attract higher compensation
Hiring for Production QA QC ADL Engineering Roles
Additional Benefits
- Performance-based incentives
- Professional training and skill development
- Exposure to regulated market manufacturing
- Supportive and collaborative work culture
- Transport and other facilities as per company policy
Why You Should Join
Finecure Pharmaceuticals is an excellent choice for professionals who want to work in a regulated, quality-driven pharmaceutical environment with exposure to international standards.
Key reasons to join include:
- USFDA and EU-GMP certified manufacturing facilities
- Strong focus on compliance, documentation, and quality
- Career growth opportunities across departments
- Stable organization with long-term vision
- Hands-on experience in OSD formulation and analytics
This hiring drive is ideal for professionals looking to strengthen their technical expertise and build a long-term career in pharmaceutical manufacturing and quality operations. Hiring for Production QA QC ADL Engineering Roles
How to Apply
Walk-In Interview Details
- Interview Date: 18th January 2026 (Sunday)
- Reporting Time: 9:30 AM to 3:00 PM
- Venue:
Finecure Pharmaceuticals Limited
A-1201, Mondeal Heights,
Near Iskcon Circle, S.G. Highway,
Ahmedabad, Gujarat
Documents to Carry
- Updated resume
- Academic certificates
- Current employment documents
- Experience letters (if applicable)
Email Application Option
Candidates who are unable to attend the walk-in interview may email their resume to:
Email ID: career@finecurepharma.com
For more information, candidates can visit the official website: www.finecurepharma.com
FAQs
Who can apply for these roles?
Candidates with relevant qualifications and 1–7 years of pharmaceutical industry experience can apply.
Is OSD experience mandatory?
OSD experience is strongly preferred, especially for Production, QA, QC, and ADL roles.
Are freshers eligible?
No. These positions are for experienced professionals only.
What is the interview mode?
This is an in-person walk-in interview.
Is Finecure Pharmaceuticals a regulated company?
Yes. Finecure is USFDA and EU-GMP certified.
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



