Hiring for Production QA QC RD Engineering at Titan Laboratories roles at Vapi for experienced pharma professionals.
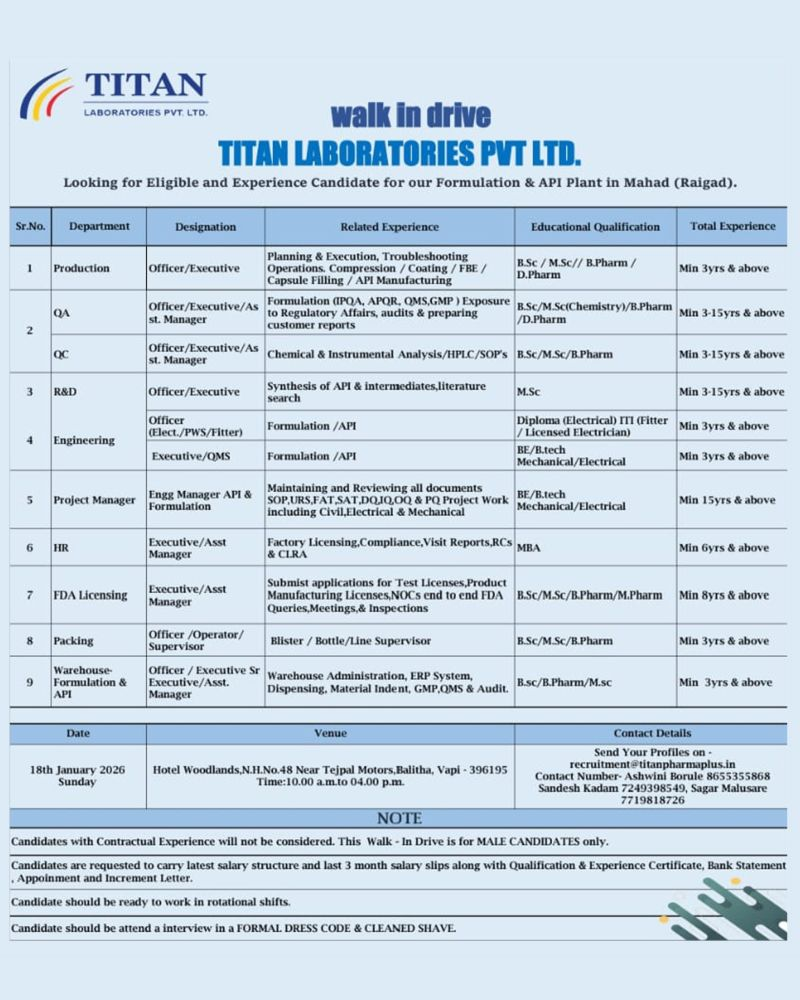
Titan Laboratories Pvt. Ltd. has announced a major walk-in interview drive scheduled for 18th January 2026 for experienced pharmaceutical professionals. The company is hiring across multiple departments including Production, Quality Assurance (QA), Quality Control (QC), Research & Development (R&D), Engineering, Packing, Warehouse, HR, and FDA Licensing.
This walk-in interview is a strong opportunity for professionals with relevant pharmaceutical experience who are looking to work in a GMP-compliant, growing organization with exposure to both formulation and API manufacturing. Hiring for Production QA QC RD Engineering
About the Company
Titan Laboratories Pvt. Ltd. is a rapidly growing, integrated pharmaceutical company with strong capabilities in formulation development and API manufacturing. The organization is well known for its expertise in sustained-release and modified-release dosage forms.
Titan Laboratories manufactures a wide range of pharmaceutical products, including pellets, granules, tablets, capsules, and active pharmaceutical ingredients (APIs). The company operates modern, GMP-compliant facilities equipped with advanced technology and strong quality systems. Hiring for Production QA QC RD Engineering
With a focus on innovation, regulatory compliance, and continuous improvement, Titan Laboratories has built a reputation for quality manufacturing and operational excellence. The company offers a professional work culture, challenging roles, and long-term career opportunities for experienced pharmaceutical professionals. Hiring for Production QA QC RD Engineering
Job Details
- Company Name: Titan Laboratories Pvt. Ltd.
- Hiring Type: Walk-in Interview
- Walk-in Date: 18th January 2026
- Job Location: Mahad, Raigad (Formulation & API Plant)
- Interview Location: Vapi, Gujarat
- Work Mode: On-site
- Shift Requirement: Rotational shifts
- Experience Required: Minimum 3 years (up to 15+ years depending on role)
- Eligibility: Male candidates only
- Important Note: Candidates with contractual experience will not be considered
Job Description
Titan Laboratories is conducting a walk-in interview to strengthen its workforce at the Formulation and API manufacturing plant in Mahad, Raigad. The company is hiring experienced professionals across core pharmaceutical functions, technical support, compliance, and operations. Hiring for Production QA QC RD Engineering
These roles involve hands-on responsibilities in regulated manufacturing environments, exposure to audits, documentation, quality systems, and advanced pharmaceutical processes. Senior-level roles include leadership, project execution, and regulatory coordination responsibilities.
Candidates must be ready to work in a GMP-compliant environment and follow strict quality, safety, and documentation standards. Hiring for Production QA QC RD Engineering
Department-wise Open Positions
Production Department
Designation: Officer / Executive
Qualification: B.Sc., M.Sc., B.Pharm, D.Pharm
Experience: Minimum 3+ years
Key Areas:
- Planning and execution of production activities
- Compression, coating, FBE, capsule filling
- API manufacturing operations
- Process troubleshooting and yield optimization
Quality Assurance (QA)
Designation: Officer / Executive / Assistant Manager
Qualification: B.Sc., M.Sc. (Chemistry), B.Pharm, D.Pharm
Experience: 3–15 years
Key Areas:
- Regulatory compliance and audit handling
- IPQA, APQR, QMS implementation
- Customer audits and regulatory inspections
- Documentation review and GMP compliance
Hiring for Production QA QC RD Engineering
Quality Control (QC)
Designation: Officer / Executive / Assistant Manager
Qualification: B.Sc., M.Sc., B.Pharm
Experience: 3–15 years
Key Areas:
- Chemical and instrumental analysis
- Operation of HPLC and other analytical instruments
- SOP preparation and method compliance
- Stability and routine QC testing
Hiring for Production QA QC RD Engineering
Research and Development (R&D)
Designation: Officer / Executive
Qualification: M.Sc.
Experience: 3–15 years
Key Areas:
- API and intermediate synthesis
- Process development and optimization
- Literature search and technical documentation
Engineering – Maintenance
Designation: Officer (Electrical / PWS / Fitter)
Qualification: Diploma (Electrical) / ITI (Fitter or Licensed Electrician)
Experience: Minimum 3+ years
Key Areas:
- Maintenance of formulation and API plant equipment
- Preventive and breakdown maintenance
- Utility systems and electrical operations
Hiring for Production QA QC RD Engineering
Engineering – QMS
Designation: Executive / QMS
Qualification: BE / B.Tech (Mechanical or Electrical)
Experience: Minimum 3+ years
Key Areas:
- Engineering quality management systems
- Documentation and compliance support
- Formulation and API plant QMS activities
Engineering – Projects
Designation: Project Manager / Engineering Manager
Qualification: BE / B.Tech (Mechanical or Electrical)
Experience: Minimum 15+ years
Key Areas:
- Project planning and execution
- Documentation including SOPs, URS, FAT, SAT
- DQ, IQ, OQ, PQ activities
- Civil, electrical, and mechanical project handling
Human Resources (HR)
Designation: Executive / Assistant Manager
Qualification: MBA
Experience: Minimum 6+ years
Key Areas:
- Factory licensing and statutory compliance
- Handling regulatory visits and reports
- RCS and CLRA compliance
FDA Licensing
Designation: Executive / Assistant Manager
Qualification: B.Sc., M.Sc., B.Pharm, M.Pharm
Experience: Minimum 8+ years
Key Areas:
- Product and test license management
- NOCs and FDA correspondence
- Regulatory meetings and inspections
Packing Department
Designation: Officer / Operator / Supervisor
Qualification: B.Sc., M.Sc., B.Pharm
Experience: Minimum 3+ years
Key Areas:
- Blister and bottle packing
- Line supervision and documentation
- GMP compliance in packing operations
Hiring for Production QA QC RD Engineering
Warehouse
Designation: Officer / Executive / Senior Executive / Assistant Manager
Qualification: B.Sc., B.Pharm, M.Sc.
Experience: Minimum 3+ years
Key Areas:
- Warehouse administration
- ERP systems and material management
- Dispensing, audits, GMP and QMS compliance
Skills and Qualifications
- Hands-on experience in formulation or API manufacturing
- Strong understanding of GMP, QMS, and regulatory compliance
- Proficiency in HPLC, analytical testing, or API synthesis
- Experience in troubleshooting, audits, and scale-up activities
- Knowledge of licensing, documentation, and regulatory standards
- Familiarity with ERP systems and cross-functional coordination
- Willingness to work in rotational shifts
Hiring for Production QA QC RD Engineering
Key Responsibilities
- Execute and monitor production and packing activities
- Perform quality control testing and documentation
- Ensure GMP compliance across departments
- Handle audits, inspections, and regulatory interactions
- Maintain equipment and manage engineering operations
- Execute and monitor API synthesis and R&D activities
- Support warehouse operations and material control
- Adhere strictly to safety, quality, and compliance standards
Benefits and Perks
- Competitive salary packages based on experience
- Clear career progression and internal growth opportunities
- Exposure to advanced formulation and API technologies
- Continuous learning and skill development
- Professional and collaborative work environment
- Long-term job stability in a growing organization
Hiring for Production QA QC RD Engineering
Walk-in Interview Details
- Date: 18th January 2026 (Sunday)
- Time: 10:00 AM to 04:00 PM
- Venue: Hotel Woodlands, N.H. No. 48, Near Tejpal Motors, Balitha, Vapi – 396195
Documents Required:
- Latest salary structure
- Last 3 months salary slips
- Qualification and experience certificates
- Bank statement
- Appointment and increment letters
Dress Code: Formal attire is mandatory. Clean shave required.
Why You Should Join Titan Laboratories
Titan Laboratories offers a professional and quality-driven environment where employees gain exposure to advanced pharmaceutical technologies and regulated manufacturing systems. The company emphasizes innovation, compliance, and teamwork. Hiring for Production QA QC RD Engineering
Working at Titan Laboratories provides professionals with opportunities to handle challenging roles, grow technically and managerially, and contribute to the development of high-quality pharmaceutical products that support healthcare needs. Hiring for Production QA QC RD Engineering
FAQs
Who is eligible for this walk-in interview?
Only male candidates with a minimum of 3 years of relevant pharmaceutical experience are eligible. Contractual experience is not accepted.
Is rotational shift work mandatory?
Yes, selected candidates must be willing to work in rotational shifts.
What documents should candidates bring?
Candidates must bring salary slips, qualification and experience certificates, bank statements, and appointment or increment letters.
Can candidates apply if they cannot attend the walk-in?
Yes, candidates who are unable to attend can apply by email.
How to Apply
Candidates who are unable to attend the walk-in interview can send their updated resume to recruitment@titanpharmaplus.in.
Mention the department name and designation clearly in the subject line.
For walk-in related queries, candidates may contact:
- Ashwini Borule – 8655355868
- Sandesh Kadam – 7249398549
- Sagar Malusare – 7719818726
Attend the walk-in interview on 18th January 2026 and take the next step in your pharmaceutical career with Titan Laboratories.
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



