Safety PV Coordinator Job in Hyderabad at Syneos Health for pharmacovigilance operations, safety case support, and clinical development roles.
About the Company
Syneos Health® is a leading, fully integrated biopharmaceutical solutions organization dedicated to accelerating customer success and improving patient outcomes worldwide. By combining deep expertise in clinical development, medical affairs, and commercial solutions, Syneos Health supports pharmaceutical, biotechnology, and medical device companies throughout the entire product lifecycle.
Operating across more than 110 countries with a workforce of approximately 29,000 employees, Syneos Health has played a critical role in the development of the majority of novel FDA-approved drugs and EMA-authorized products in recent years. The organization is widely respected for its innovative Clinical Development model, which places both the customer and the patient at the center of everything it does. Safety PV Coordinator Job in Hyderabad
Syneos Health is known for its agile mindset, collaborative culture, and strong commitment to employee development. The company continuously invests in training, career progression, and employee well-being, creating an environment where professionals can grow while contributing to life-changing therapies. Safety PV Coordinator Job in Hyderabad
Job Details
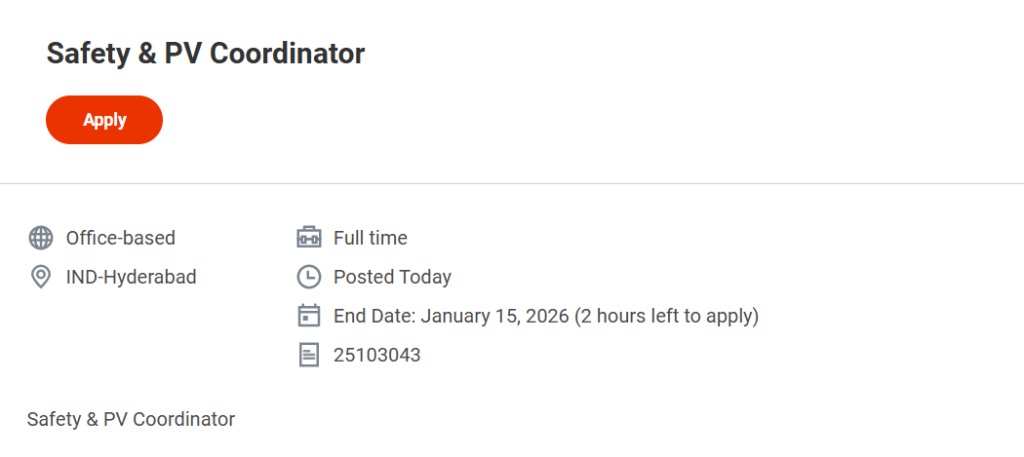
- Job Title: Safety & PV Coordinator
- Job Requisition ID: 25103043
- Company: Syneos Health®
- Employment Type: Full-Time
- Work Mode: Office-Based
- Job Location: Hyderabad, India
- Department: Pharmacovigilance / Safety
- Application Deadline: January 15, 2026
- Time Left to Apply: Limited (Urgent Requirement)
Job Description
Syneos Health is currently hiring a Safety & Pharmacovigilance (PV) Coordinator for its Hyderabad office. This role is part of the organization’s safety and clinical development operations and supports global pharmacovigilance activities. Safety PV Coordinator Job in Hyderabad
As a Safety & PV Coordinator, you will contribute to the effective management of safety data and pharmacovigilance processes, supporting the timely and compliant handling of safety information across clinical trials and post-marketing activities. This position is well-suited for professionals who are detail-oriented, process-driven, and interested in building a long-term career in drug safety and pharmacovigilance.
The role provides exposure to global safety operations, regulated environments, and cross-functional collaboration within a leading biopharmaceutical services organization. Safety PV Coordinator Job in Hyderabad
Key Responsibilities
Pharmacovigilance and Safety Operations Support
- Support pharmacovigilance activities in accordance with internal procedures, regulatory requirements, and global safety standards.
- Assist with the coordination of safety-related tasks across clinical development programs.
- Ensure accurate and timely processing of safety information.
Safety Data and Documentation Management
- Maintain and organize safety documentation, trackers, and databases.
- Support the management of safety data and related administrative activities.
- Ensure documentation is accurate, complete, and inspection-ready.
Safety PV Coordinator Job in Hyderabad
Compliance and Quality Support
- Support compliance with global pharmacovigilance regulations and internal SOPs.
- Assist in ensuring adherence to timelines, quality standards, and reporting requirements.
- Participate in quality checks and follow-up activities as required.
Cross-Functional Collaboration
- Work closely with safety scientists, clinical operations, and other cross-functional teams.
- Facilitate effective communication between internal stakeholders to support smooth safety operations.
- Escalate issues and risks appropriately to senior team members.
Safety PV Coordinator Job in Hyderabad
Process and Operational Support
- Support process improvement initiatives aimed at increasing efficiency and compliance.
- Assist with preparation for audits, inspections, and internal reviews when required.
- Perform additional safety-related tasks as assigned based on business needs.
Safety PV Coordinator Job in Hyderabad
Skills and Qualifications
Educational Qualifications
- Bachelor’s degree in Life Sciences, Pharmacy, Biotechnology, Clinical Research, Nursing, or a related healthcare or scientific discipline.
- Equivalent experience, skills, or education may also be considered.
Technical Knowledge and Experience
- Basic understanding of pharmacovigilance and drug safety concepts.
- Familiarity with clinical development and safety operations is an advantage.
- Knowledge of global safety regulations and guidelines is desirable.
Safety PV Coordinator Job in Hyderabad
Professional Skills
- Strong attention to detail and accuracy in data handling.
- Good organizational and time-management skills.
- Ability to work effectively in a fast-paced and regulated environment.
- Strong written and verbal communication skills.
- Ability to collaborate effectively within cross-functional teams.
Personal Attributes
- Proactive and quality-focused mindset.
- Willingness to learn and adapt in a dynamic environment.
- Commitment to ethical standards and patient safety.
Safety PV Coordinator Job in Hyderabad
Benefits and Perks
Syneos Health offers a competitive and comprehensive rewards and benefits package designed to support professional growth, well-being, and long-term career development. Benefits may vary by location but typically include: Safety PV Coordinator Job in Hyderabad
- Competitive salary aligned with industry standards.
- Structured career development and progression opportunities.
- Supportive and engaged line management.
- Technical and therapeutic area training programs.
- Peer recognition and total rewards programs.
- Strong focus on work-life balance.
- Inclusive “Total Self” culture that encourages authenticity and belonging.
- Access to global learning resources and professional development platforms.
Why You Should Join
Joining Syneos Health as a Safety & PV Coordinator offers an excellent opportunity to build or strengthen your career in pharmacovigilance within a globally recognized organization. You will gain exposure to large-scale global safety operations and contribute directly to the development and monitoring of therapies that improve patient lives.
Syneos Health is committed to developing its people, fostering diversity, and creating a workplace where every individual feels valued and supported. Whether you are early in your pharmacovigilance career or looking to grow within safety operations, this role provides meaningful learning opportunities and long-term career potential. Safety PV Coordinator Job in Hyderabad
The organization encourages employees to challenge the status quo, collaborate across functions, and continuously innovate in a highly competitive and evolving healthcare environment.
How to Apply
Interested and eligible candidates should apply as soon as possible due to the urgent application deadline.
Early application is strongly recommended as the position is time-sensitive. Safety PV Coordinator Job in Hyderabad
FAQs
What is the job location for this role?
The Safety & PV Coordinator role is based in Hyderabad, India, and is office-based.
Is this a full-time position?
Yes, this is a full-time role with Syneos Health.
What department does this role fall under?
This position is part of the Pharmacovigilance and Safety function within Clinical Development.
Is prior pharmacovigilance experience mandatory?
Prior experience is beneficial, but equivalent experience, skills, or education may also be considered.
Does Syneos Health support diversity and inclusion?
Yes, Syneos Health is committed to equal employment opportunities, diversity, inclusion, and providing reasonable accommodations where required.
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



