Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing staff with freshers eligibility.
Introduction
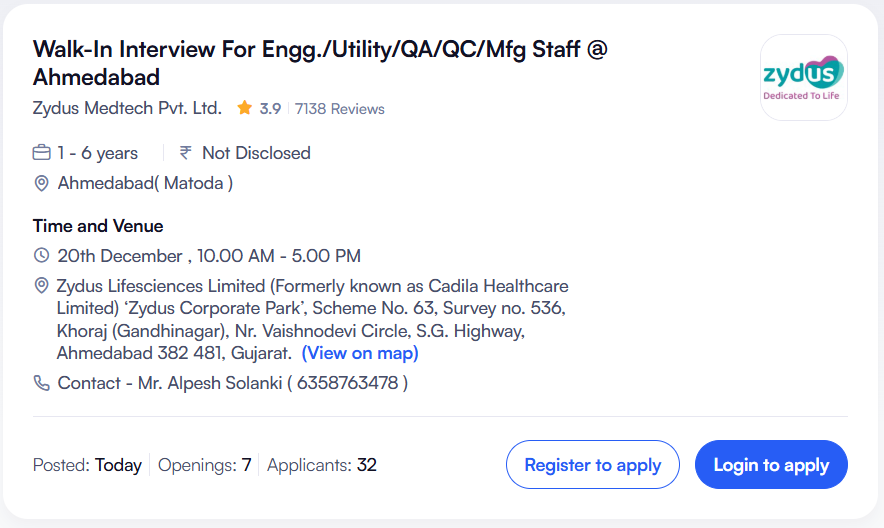
Zydus Medtech Pvt. Ltd. has announced a major walk-in interview drive in Ahmedabad for experienced professionals as well as freshers across multiple departments. This hiring initiative is for its greenfield medical device manufacturing project at Matoda, Ahmedabad, offering a rare opportunity to become part of a fast-growing and innovation-driven organization within the medical devices and healthcare technology sector. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
Zydus Medtech is a part of the renowned Zydus group, known globally for quality, compliance, and innovation. The company focuses on advanced medical technologies and manufacturing excellence, making this recruitment drive especially attractive for candidates looking for long-term career stability, exposure to world-class systems, and growth in leadership roles.
This walk-in interview is scheduled for 20th December, and candidates with qualifications ranging from B.Sc to M.Tech and B.Pharma to M.Pharma are eligible. Both freshers and experienced professionals up to 15 years of relevant experience are encouraged to participate. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
Key Highlight Box
Company Name: Zydus Medtech Pvt. Ltd.
Industry Type: Medical Devices & Equipment
Job Location: Matoda, Ahmedabad, Gujarat
Interview Type: Walk-In Interview
Date: 20th December
Time: 10:00 AM to 5:00 PM
Position Level: Officer to Senior Manager
Departments Hiring: QA, QC, RA, Microbiology, Engineering, Utility, PPC
Experience Required: Freshers to 15 Years
Employment Type: Full Time, Permanent
Qualification: B.Sc, M.Sc, B.Pharma, M.Pharma, B.Tech, B.E
Number of Openings: 7
About Zydus Medtech Pvt. Ltd.
Zydus Lifesciences Limited, formerly known as Cadila Healthcare Limited, is one of India’s most respected healthcare groups. Zydus Medtech Pvt. Ltd. operates as a specialized arm focusing on medical devices, diagnostic solutions, and advanced healthcare technologies.
With a strong emphasis on regulatory compliance, quality assurance, and patient safety, Zydus Medtech follows global standards such as ISO, GMP, and international medical device regulations. The Ahmedabad Matoda site is a greenfield project, which means selected candidates will get hands-on experience in setting up systems, processes, validations, and large-scale operations from the ground up.
Working in a greenfield project not only accelerates learning but also provides high visibility within the organization, making it ideal for ambitious professionals. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
Walk-In Interview Details
The walk-in interview will be conducted at the Zydus Corporate Park in Ahmedabad.
Venue Address:
Zydus Corporate Park,
Scheme No. 63, Survey No. 536,
Khoraj (Gandhinagar),
Near Vaishnodevi Circle,
S.G. Highway, Ahmedabad – 382481, Gujarat
Date: 20th December
Timing: 10:00 AM to 5:00 PM
Contact Person:
Mr. Alpesh Solanki
Contact Number: 6358763478
Candidates are advised to reach the venue early to complete the registration and interview process smoothly. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
Departments and Positions Available
Zydus Medtech is hiring across multiple critical departments that support medical device manufacturing, compliance, and operations. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
1. Quality Department (RA, QA, QC & Microbiology)
The Quality Department plays a vital role in ensuring that products meet national and international regulatory standards. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
Key Responsibilities:
- Regulatory Affairs documentation and submissions
- Quality Assurance system implementation and compliance
- Quality Control testing and analysis
- Microbiological monitoring and environmental control
- SOP preparation, review, and implementation
- Handling audits, inspections, and regulatory queries
- Batch release and product lifecycle management
Candidates with exposure to medical devices, pharmaceuticals, or regulated manufacturing environments will have an added advantage.
2. Engineering Services
Engineering professionals will be responsible for ensuring smooth operation and maintenance of production equipment.
Key Responsibilities:
- Preventive and breakdown maintenance of process equipment
- Equipment qualification and validation support
- Calibration and documentation activities
- Coordination with production and quality teams
- Utility and machinery performance optimization
This role is crucial for maintaining uptime and ensuring compliance with manufacturing standards.
3. Utility Services
Utility professionals ensure uninterrupted plant operations and facility management.
Key Responsibilities:
- Operation and maintenance of boilers, DG sets, compressors, and chillers
- Handling HVAC systems and cleanroom utilities
- Water systems including RO, WFI, and purified water
- Monitoring energy efficiency and safety compliance
- Documentation and audit readiness
Candidates with experience in regulated industries such as pharma or medical devices will be preferred. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
4. Production Planning and Control (PPC)
The PPC department ensures efficient production scheduling and supply chain coordination.
Key Responsibilities:
- Procurement of raw materials
- Production planning and scheduling
- Inventory control and material management
- Coordination with vendors and internal departments
- Dispatch planning for domestic and export shipments
- ERP and documentation management
This role is ideal for candidates who enjoy analytical planning and cross-functional coordination. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
Eligibility Criteria
Educational Qualifications:
- B.Sc / M.Sc (Any Specialization)
- B.Pharma / M.Pharma
- B.Tech / B.E (Any Engineering Discipline)
Experience:
- Freshers may be considered as per company norms
- Experienced candidates up to 15 years of relevant industry experience preferred
Position Levels:
- Officer
- Executive
- Manager
- Senior Manager
Skills and Competencies Preferred
- Knowledge of GMP and regulatory guidelines
- Strong documentation and compliance skills
- Hands-on experience in regulated manufacturing environments
- Good communication and coordination abilities
- Problem-solving and analytical mindset
- Willingness to work in a greenfield project setup
Why Join Zydus Medtech?
Joining Zydus Medtech offers several long-term career benefits: Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
- Opportunity to work on a greenfield medical device project
- Exposure to international quality and regulatory standards
- Stable and reputed organization under the Zydus Group
- Learning-oriented work culture
- Career growth from officer to leadership levels
- Permanent employment with structured systems
This walk-in drive is particularly valuable for professionals seeking career acceleration and hands-on industrial exposure.
Important Instructions for Candidates
- Carry updated resume copies
- Bring original and photocopies of educational certificates
- Carry experience letters and salary slips, if applicable
- Carry government-issued ID proof
- Dress in formal attire
- Be prepared for technical and HR discussions
How to Apply
Interested candidates should directly attend the walk-in interview on the scheduled date and time. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
Walk-In Date: 20th December
Time: 10:00 AM to 5:00 PM
Venue: Zydus Corporate Park, Ahmedabad
For queries, candidates may contact:
Mr. Alpesh Solanki – 6358763478
No prior online application is mandatory. Selection will be based on interview performance and eligibility as per company norms. Zydus Medtech Hiring for Engineering Utility QA QC Manufacturing
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



