Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts roles through walk-in interviews in Mehsana, Gujarat.
Introduction
Anbuva Pharma Pvt. Ltd., a rapidly growing pharmaceutical company engaged in the manufacturing of Active Pharmaceutical Ingredients (APIs) and Intermediates, has announced a large-scale recruitment drive for multiple technical and non-technical positions. This hiring initiative is aimed at strengthening its operational, quality, safety, and administrative teams at its advanced manufacturing facility located in Mehsana, Gujarat. Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts
The company is conducting walk-in interviews on 15th and 16th December 2025, offering an excellent opportunity for experienced professionals from the pharmaceutical and API manufacturing sector. Candidates with relevant educational backgrounds and hands-on experience in Production, Quality Control, Quality Assurance, Microbiology, Engineering, Warehouse, EHS, HR, and Accounts are encouraged to attend.
With its strong adherence to WHO-GMP and ISO standards, Anbuva Pharma continues to expand its footprint in domestic and international pharmaceutical markets. This recruitment drive is ideal for professionals seeking stable career growth, exposure to regulated environments, and long-term opportunities in the API industry. Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts
About Anbuva Pharma Pvt. Ltd.
Anbuva Pharma Pvt. Ltd. is a reputed manufacturer specializing in APIs and pharmaceutical intermediates. The company operates with a strong quality-driven approach, ensuring compliance with global regulatory requirements. Its manufacturing facility in Mehsana is equipped with modern infrastructure, advanced process equipment, and robust quality systems. Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts
Anbuva Pharma supplies high-quality products to regulated and semi-regulated markets and follows strict guidelines related to Good Manufacturing Practices (GMP), quality assurance, environmental safety, and occupational health. The organization believes in continuous improvement, employee development, and sustainable manufacturing practices. Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts
Key Highlights – Anbuva Pharma Walk-In Drive 2025
Company Name: Anbuva Pharma Pvt. Ltd.
Industry: API & Intermediates Manufacturing
Job Type: Full-time, On-roll Positions
Interview Type: Walk-In Interview
Interview Dates: 15th & 16th December 2025
Job Location: Mehsana, Gujarat
Experience Required: 1 to 5 years (varies by role)
Education: B.Sc, M.Sc, B.Pharm, M.Pharm, Diploma, BE, B.Com, BBA, MBA, Graduation
Salary Range: INR 3,00,000 to 7,00,000 per annum
Compliance Standards: WHO-GMP, ISO
Departments Hiring: Production, QC, QA, Microbiology, Engineering, Warehouse, EHS, HR, Accounts
Available Positions and Eligibility Criteria
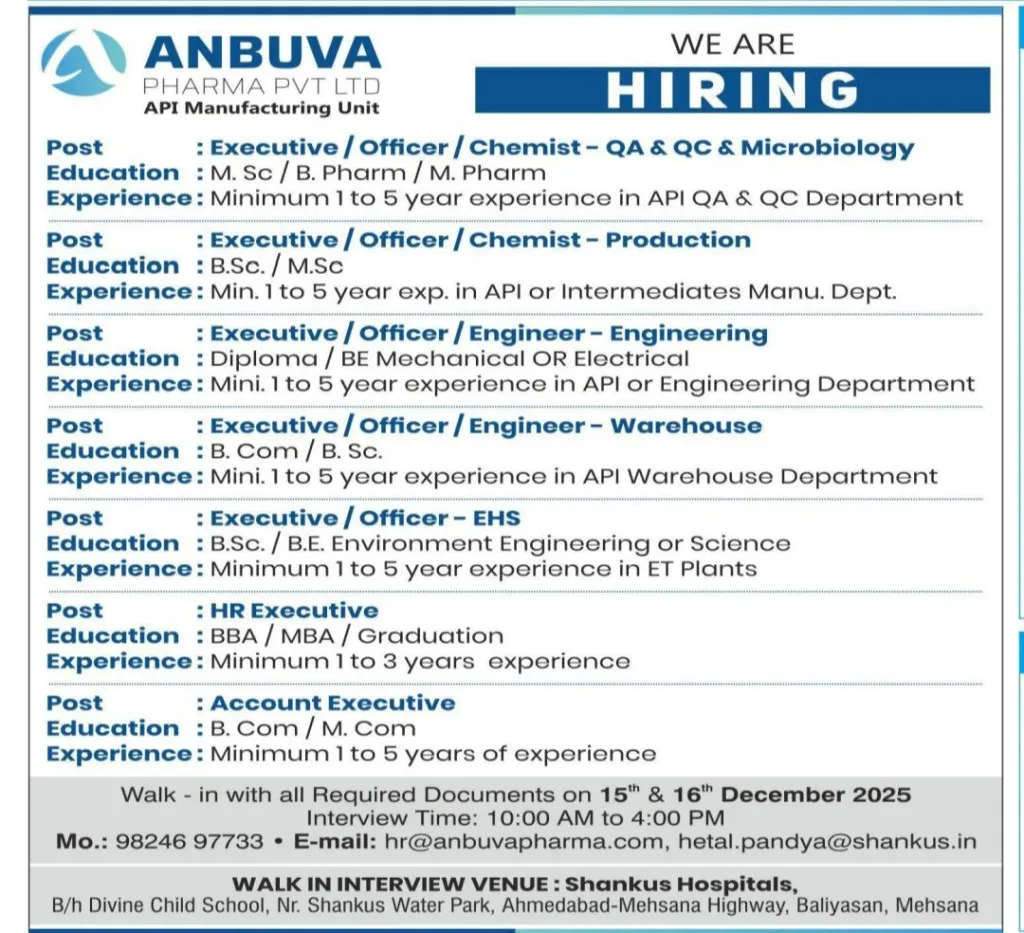
1. Executive / Officer / Chemist – QA, QC & Microbiology
Educational Qualification:
M.Sc, B.Pharm, or M.Pharm
Experience Required:
1 to 5 years of relevant experience in API Quality Assurance, Quality Control, or Microbiology departments.
Job Overview:
Candidates will be responsible for ensuring product quality, regulatory compliance, documentation accuracy, and laboratory operations as per GMP standards.
2. Executive / Officer / Chemist – Production
Educational Qualification:
B.Sc or M.Sc (Chemistry or related discipline)
Experience Required:
1 to 5 years of experience in API or pharmaceutical intermediates manufacturing.
Job Overview:
Production professionals will manage batch manufacturing processes, operate reactors and equipment, follow SOPs, and meet production targets while maintaining quality and safety standards.
3. Executive / Officer / Engineer – Engineering
Educational Qualification:
Diploma or BE in Mechanical or Electrical Engineering
Experience Required:
1 to 5 years of experience in API plant engineering or pharmaceutical utilities.
Job Overview:
Responsibilities include maintenance of equipment, utilities management, breakdown handling, preventive maintenance, and ensuring uninterrupted plant operations.
4. Executive / Officer / Engineer – Warehouse
Educational Qualification:
B.Com or B.Sc
Experience Required:
1 to 5 years of experience in API or pharmaceutical warehouse operations.
Job Overview:
Candidates will handle raw material and finished goods inventory, documentation, SAP entries, material dispensing, and compliance with GMP warehouse practices.
5. Executive / Officer – EHS (Environment, Health & Safety)
Educational Qualification:
B.Sc or B.E in Environmental Engineering or Environmental Science
Experience Required:
1 to 5 years of experience in ETP plants or pharmaceutical EHS roles.
Job Overview:
The EHS executive will oversee safety compliance, waste management, effluent treatment plant operations, risk assessments, and statutory environmental requirements.
6. HR Executive
Educational Qualification:
BBA, MBA, or Graduation in relevant discipline
Experience Required:
1 to 3 years of experience in HR operations.
Job Overview:
HR executives will manage recruitment, onboarding, employee relations, attendance, payroll coordination, and HR documentation.
7. Account Executive
Educational Qualification:
B.Com or M.Com
Experience Required:
1 to 5 years of experience in accounting or finance.
Job Overview:
Responsibilities include handling accounts, billing, taxation, ledger maintenance, audits, and financial documentation.
Roles and Responsibilities (General)
Selected candidates will be expected to contribute to the organization’s operational excellence and regulatory compliance. Key responsibilities across departments include:
- Ensuring adherence to WHO-GMP, ISO, and internal quality systems
- Maintaining accurate documentation and records
- Supporting audits, inspections, and regulatory requirements
- Following SOPs, safety guidelines, and environmental norms
- Coordinating with cross-functional teams for smooth operations
- Achieving departmental targets with a focus on quality and efficiency
- Continuous improvement and compliance-driven performance
Required Skills and Professional Expectations
- Strong knowledge of GMP and pharmaceutical regulations
- Hands-on experience in API or pharmaceutical manufacturing environments
- Attention to detail and documentation accuracy
- Ability to work in regulated and audited environments
- Good communication and teamwork skills
- Willingness to learn and adapt to evolving industry standards
Salary and Benefits
Anbuva Pharma offers competitive salary packages based on experience, skill set, and role requirements. The annual compensation typically ranges between INR 3,00,000 to 7,00,000, depending on position and experience level. Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts
Additional benefits include:
- Provident Fund (PF)
- Health and medical insurance
- Performance-based incentives
- Stable work environment
- Career growth and learning opportunities
- Exposure to regulated API manufacturing
Why Join Anbuva Pharma?
- Opportunity to work in a WHO-GMP compliant API manufacturing facility
- Exposure to international quality standards
- Career stability in a growing pharmaceutical organization
- Learning-driven work culture
- Professional development and long-term growth prospects
How to Apply (Walk-In Interview Details)
Interested and eligible candidates are required to attend the walk-in interview in person with updated resumes and relevant documents. Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts
Interview Dates:
15th December 2025
16th December 2025
Interview Time:
10:00 AM to 4:00 PM
Interview Venue:
Shankus Hospitals
Behind Divine Child School
Near Shankus Water Park
Ahmedabad–Mehsana Highway
Baliyasan, Mehsana, Gujarat
Documents to Carry:
- Updated Resume
- Educational Certificates
- Experience Certificates
- ID Proof
- Passport Size Photographs
Contact Details:
Mobile: 98246 97733
Email: hr@anbuvapharma.com
Alternate Email: hetal.pandya@shankus.in
This walk-in drive by Anbuva Pharma Pvt. Ltd. is a valuable opportunity for professionals seeking to advance their careers in the pharmaceutical and API manufacturing industry. Candidates meeting the eligibility criteria are advised to attend the interview on the scheduled dates without delay. Anbuva Pharma Hiring for Production QC QA Microbiology Warehouse Engineering EHS HR Accounts
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



