Ami Lifesciences Hiring for Production / Engineering / QC professionals in Vadodara. Walk-in Dec 5, 2025. Apply with resume and payslips
Ami Lifesciences Walk-In Interview 2025 — Production, Engineering & Quality Control Roles | Vadodara
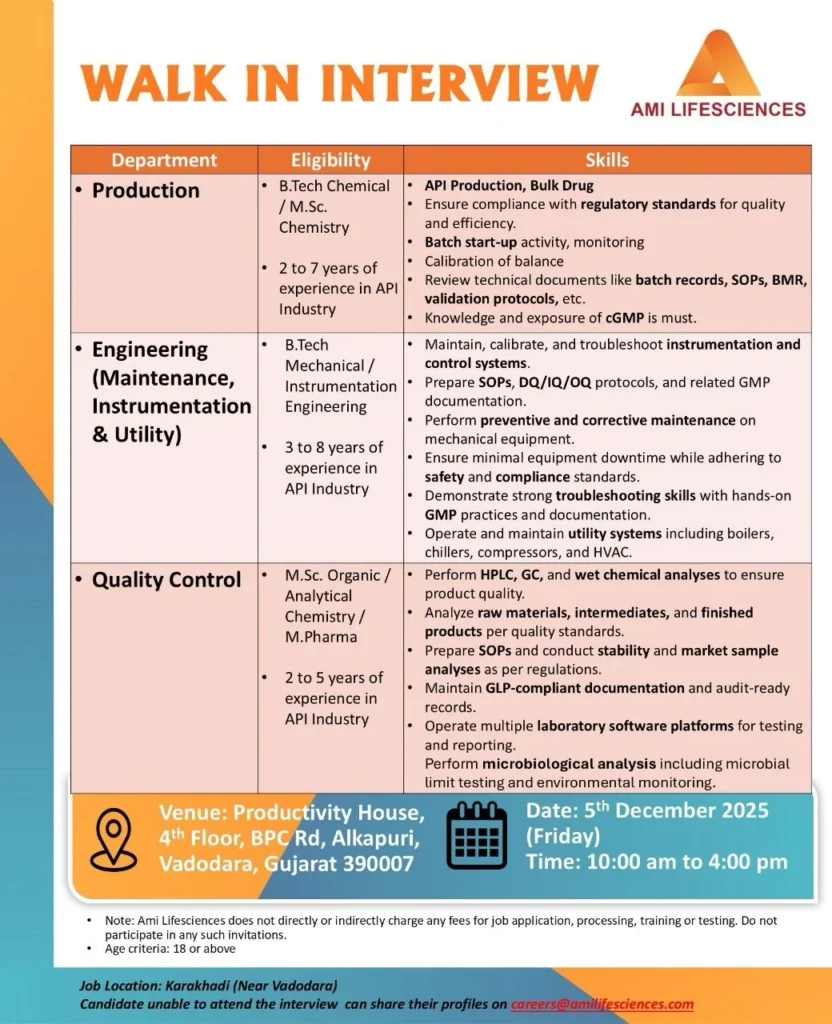
Ami Lifesciences, a rapidly expanding API and bulk drug manufacturer, is hosting a mega walk-in interview in Vadodara on Friday, 5 December 2025 for experienced professionals across Production, Engineering (Maintenance/Instrumentation/Utility) and Quality Control (QC). If you have 2–8 years of hands-on experience in the API/pharma sector and want to move into a stable, growth-oriented manufacturing environment near Vadodara, this event is designed for you. Below is a comprehensive, SEO-friendly job post with role details, responsibilities, eligibility, salary range, benefits, and clear application instructions. Ami Lifesciences Hiring for Production / Engineering / QC
Key Highlights
| Category | Details |
|---|---|
| Company | Ami Lifesciences |
| Recruitment Type | Walk-in Interview |
| Date & Time | 5 December 2025, 10:00 AM – 4:00 PM |
| Venue | Productivity House, 4th Floor, BPC Road, Alkapuri, Vadodara, Gujarat 390007 |
| Job Location | Karakhadi (near Vadodara) |
| Departments | Production, Engineering (Maintenance/Instrumentation/Utility), Quality Control |
| Qualifications | B.Tech, M.Sc, M.Pharm (as applicable per role) |
| Experience | 2–8 years (role-specific) |
| Salary Range (CTC) | ₹3.6 LPA – ₹9.0 LPA (depending on experience & position) |
| Application Mode | Walk-in / Email (careers@amilifesciences.com) |
| Fee | No application fee; company does NOT charge any fees |
About Ami Lifesciences
Ami Lifesciences is a fast-growing Indian manufacturer specializing in Active Pharmaceutical Ingredients (APIs) and bulk drugs. The company serves domestic and international markets and emphasizes quality, regulatory compliance, and process excellence. Ami’s manufacturing units follow cGMP standards and regularly prepare for regulatory audits. The Vadodara walk-in drive is part of Ami’s focused hiring to strengthen manufacturing, engineering, and analytical capabilities at its Karakhadi facility. Ami Lifesciences Hiring for Production / Engineering / QC
Open Positions & Eligibility
| Department | Qualification | Experience Required |
|---|---|---|
| Production (API / Bulk Drugs) | B.Tech (Chemical) / M.Sc. (Chemistry) | 2–7 years |
| Engineering (Maintenance / Instrumentation / Utility) | B.Tech (Mechanical / Instrumentation) | 3–8 years |
| Quality Control (QC) | M.Sc. Organic / Analytical Chemistry / M.Pharm | 2–5 years |
Note: Exact experience ranges vary by department. Candidates must have relevant API or bulk-drug industry experience. Ami Lifesciences Hiring for Production / Engineering / QC
Role Responsibilities (What You Will Do)
Production (API / Bulk Drugs)
- Manage batch start-up, monitoring, and process optimization for routine and non-routine batches.
- Ensure strict adherence to cGMP and safety standards.
- Maintain accurate Batch Manufacturing Records (BMR) and documentation.
- Coordinate with QA to resolve deviations and support process validation activities.
- Assist in troubleshooting process bottlenecks and implement corrective actions.
Ami Lifesciences Hiring for Production / Engineering / QC
Engineering — Maintenance / Instrumentation / Utility
- Plan and execute preventive and breakdown maintenance for mechanical equipment.
- Calibrate and troubleshoot instrumentation and control systems (PLC/DCS interfaces).
- Operate and maintain plant utilities: boilers, chillers, HVAC, compressed air systems.
- Prepare technical documentation: SOPs, DQ/IQ/OQ/PQ protocols and maintenance logs.
- Support continuous improvement and reliability programs to improve OEE.
Ami Lifesciences Hiring for Production / Engineering / QC
Quality Control (QC)
- Perform routine and non-routine analytical testing: HPLC, GC, UV, titrations, wet chemistry.
- Execute stability studies, environmental monitoring, and microbiological testing where applicable.
- Maintain GLP/CGMP-compliant records and prepare for internal and external audits.
- Support method validation, instrument qualification, and troubleshooting.
- Work closely with Production and QA for sample management and release decisions.
Ami Lifesciences Hiring for Production / Engineering / QC
What Ami Lifesciences Offers
- Competitive salary aligned with experience and industry benchmarks.
- Timely payouts and statutory benefits.
- Exposure to regulatory audits (USFDA / EU-GMP readiness) and strong compliance culture.
- Career growth opportunities in a rapidly expanding API manufacturing setup.
- On-site conveniences such as free/paid transport and subsidized canteen (where applicable).
- Hands-on technical learning across process development, instrumentation, and analytical techniques.
Salary Range (Indicative): ₹3.6 LPA – ₹9.0 LPA depending on department, seniority, and experience. Ami Lifesciences Hiring for Production / Engineering / QC
Walk-In Interview Details & How to Apply
Date: Friday, 5 December 2025
Time: 10:00 AM – 4:00 PM (report early to avoid queues)
Venue: Productivity House, 4th Floor, BPC Road, Alkapuri, Vadodara, Gujarat 390007
Job Location: Karakhadi (near Vadodara)
Walk-In Process: Direct spot interviews will be conducted. Carry original documents for verification. Candidates who cannot attend in person may email their resume to: careers@amilifesciences.com with subject line: “Application for [Department] – Vadodara Walk-In Dec 2025”. Ami Lifesciences Hiring for Production / Engineering / QC
Important: Ami Lifesciences does NOT charge any fee for recruitment or documentation at any stage. Do not pay or respond to requests for payment.
Ami Lifesciences Hiring for Production / Engineering / QC
Documents to Carry (Originals + One Set Photocopy)
- Updated resume / CV
- Recent passport-size photograph
- Last 3 months’ payslips (bring salary slips or salary certificate)
- Educational certificates (degree/diploma/mark sheets)
- Experience letters / relieving letters from previous employers
- Aadhaar Card / PAN Card / Voter ID (proof of identity)
- Any professional certificates (e.g., instrument training, safety training)
Interview Preparation Tips
- Refresh fundamentals relevant to your domain (HPLC/GC theory for QC; unit operations and process chemistry for Production; preventive maintenance and control systems for Engineering).
- Prepare concise, fact-based examples of how you solved technical or production-related problems at previous roles.
- Be ready to discuss SOPs, cGMP practices, BMR handling, and deviation/CAPA examples.
- Carry multiple copies of your resume and neatly organize documents in a folder.
- Dress formally; present a professional and punctual appearance.
- Ami Lifesciences Hiring for Production / Engineering / QC
Frequently Asked Questions (FAQ)
Q: Can freshers apply?
A: No. This walk-in is specifically for professionals with 2–8 years of relevant API/bulk drug experience.
Q: Is online registration required?
A: No prior registration is needed for walk-in. Candidates who cannot attend can email their CV to careers@amilifesciences.com.
Q: Will the company charge any recruitment fee?
A: No. Ami Lifesciences does not charge any fee at any recruitment stage.
Q: What is the selection process?
A: Typical flow: Document verification → Technical interview (departmental) → HR round → Offer (subject to background and reference checks).
Q: Are relocation or joining bonuses offered?
A: Offer details including relocation assistance, if any, will be communicated individually based on role and negotiation.
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.


