Sun Pharma Hiring for Executive Regulatory Affairs, eligibility, and application process for candidates seeking regulatory careers.
Sun Pharma Recruitment 2025: Executive – Regulatory Affairs (R&D), Baroda | Full Job Details, Responsibilities, Eligibility and Application Process
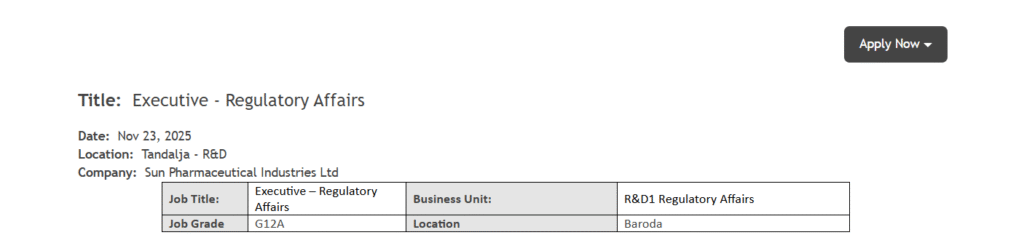
Sun Pharmaceutical Industries Ltd, one of the world’s leading specialty generic pharmaceutical companies, is inviting applications for the position of Executive – Regulatory Affairs. This opening is based at the company’s Tandalja – R&D location in Baroda and falls under the R&D1 Regulatory Affairs Business Unit with Job Grade G12A. This role offers an opportunity for regulatory professionals to work within a highly advanced research and development environment while contributing to global market submissions. Sun Pharma Hiring for Executive Regulatory Affairs
Sun Pharma emphasizes empowering its employees with the confidence and clarity to build a strong career path. The organization’s value-driven culture encourages every individual to grow at their own pace, take responsibility for their professional journey, and collaborate within a supportive and knowledgeable community. The company strongly believes in helping each employee become better every day by fostering an environment of continuous improvement, active leadership, and united success. Sun Pharma Hiring for Executive Regulatory Affairs
For candidates who are ambitious, detail-oriented, and passionate about regulatory science, this role presents an excellent opportunity to work on regulatory submissions, lifecycle management, and compliance for multiple international markets, especially across the MENA region. Sun Pharma Hiring for Executive Regulatory Affairs
About the Role: Executive – Regulatory Affairs
The Executive in Regulatory Affairs will be responsible for regulatory submissions of new pharmaceutical products, renewals, variations, and lifecycle updates for the MENA markets, which include GCC, UAE, Oman, Saudi Arabia, Egypt, Iran, Iraq, Bahrain, and other neighboring regions. The role requires a deep understanding of CMC documentation, regulatory guidelines, country-specific requirements, and strong coordination with cross-functional teams to ensure timely approvals. Sun Pharma Hiring for Executive Regulatory Affairs
This role is ideal for professionals with 1–4 years of experience in regulatory affairs and who possess a strong background in M.Sc or M.Pharm with exposure to dossier preparation, product lifecycle management, and regulatory communication. Sun Pharma Hiring for Executive Regulatory Affairs
Job Description and Key Responsibilities
This position demands a combination of technical expertise, documentation excellence, regulatory knowledge, and thorough understanding of market-specific compliance standards. The role covers end-to-end responsibilities from new filings to lifecycle management. Sun Pharma Hiring for Executive Regulatory Affairs
Below is a detailed breakdown: Sun Pharma Hiring for Executive Regulatory Affairs
1. New Submissions and Renewals
The candidate will be involved in all stages of preparing Chemistry, Manufacturing, and Controls (CMC) dossiers for new product submissions as well as renewals. Responsibilities include: Sun Pharma Hiring for Executive Regulatory Affairs
a. CMC Dossier Preparation
Preparing high-quality CMC dossiers for fresh submissions is a key part of this role. This involves reviewing and compiling technical documents, manufacturing processes, specifications, stability data, control strategies, and other essential regulatory information required for MENA submissions.
b. Pre-Exhibit Batch Review
Before exhibit batches are initiated, the Executive must thoroughly review the development report, scale-up report, artwork, stability protocol, and specifications. Ensuring the documents are complete and accurate helps prevent regulatory issues during or after submission.
2. Handling Approvals and Deficiency Responses
Regulatory authorities often raise queries or point out deficiencies during dossier evaluation. The candidate must: Sun Pharma Hiring for Executive Regulatory Affairs
- Prepare comprehensive responses to regulatory queries
- Coordinate with internal teams to obtain clarifications
- Address all deficiencies in a timely manner
- Ensure faster approval of filed products
This step requires strong communication skills, technical knowledge, and the ability to interpret regulatory feedback effectively.
3. Lifecycle Management of Drug Formulations
Lifecycle management is one of the most critical aspects of regulatory affairs. Responsibilities include: Sun Pharma Hiring for Executive Regulatory Affairs
a. Variations Preparation
The candidate must review and prepare variations according to the country-specific regulations. These updates may involve several types of changes, including:
- API vendor change
- Change in route of synthesis
- Updates in test parameters
- Drug formulation site changes
- Harmonization of product details across regions
Ensuring accuracy of variation data is crucial for smooth regulatory approvals. Sun Pharma Hiring for Executive Regulatory Affairs
4. Regulatory Compliance and Documentation Control
Compliance is central to regulatory affairs. The candidate will manage approval packages and compliance documentation, including:
a. Approval Package Preparation and Circulation
Upon receiving approval from a regulatory agency, the Executive must prepare and circulate the approval package along with a product history sheet to relevant stakeholders.
b. Updating Regulatory Repository
All product information must be accurately repositioned into the central regulatory repository for future reference.
c. Change Control Review
Each change control, variation, or update must be analyzed for regulatory impact. The Executive will determine filing requirements and ensure all documentation aligns with regulatory guidelines.
5. Geographic Scope of Responsibilities
This position handles regulatory submissions and updates for the following countries:
- GCC Region
- UAE
- Oman
- Saudi Arabia
- Egypt
- Iran
- Iraq
- Bahrain
Knowledge of MENA regulatory frameworks and submission gateways is a strong advantage. Sun Pharma Hiring for Executive Regulatory Affairs
Travel Requirement
This position does not require any travel. All responsibilities are office-based within the R&D facility. Sun Pharma Hiring for Executive Regulatory Affairs
Job Requirements
Educational Qualification
- M.Sc (Master of Science)
- M.Pharm (Master of Pharmacy)
Experience
- Required experience: 1 to 4 years in Regulatory Affairs
- Experience in CMC dossier preparation is desirable
- Exposure to MENA region regulations is an added advantage
Your Success Matters at Sun Pharma
Sun Pharma is known for nurturing talent, promoting innovation, and creating strong career pathways. The company provides excellent opportunities for professional growth, a dynamic working environment, and a culture rooted in responsibility and collaboration. Sun Pharma Hiring for Executive Regulatory Affairs
Whether you are starting your regulatory career or seeking growth, this role offers a solid platform to learn, grow, and contribute to global pharmaceutical regulatory processes. Sun Pharma Hiring for Executive Regulatory Affairs
Key Box: Highlights of the Job
| Feature | Details |
|---|---|
| Job Title | Executive – Regulatory Affairs |
| Location | Tandalja R&D, Baroda |
| Department | R&D1 Regulatory Affairs |
| Education | M.Sc / M.Pharm |
| Experience | 1–4 years |
| Markets Handled | MENA (GCC, UAE, Oman, Saudi Arabia, Egypt, Iran, Iraq, Bahrain) |
| Key Role | CMC Dossier Preparation, Variations, Lifecycle Management |
| Travel | Not Applicable |
| Company | Sun Pharmaceutical Industries Ltd |
How to Apply for Sun Pharma Executive – Regulatory Affairs Job
Interested and eligible candidates can apply through the official Sun Pharma careers portal. Ensure your resume highlights:
- Experience in regulatory submissions
- Exposure to CMC documentation
- Communications with regulatory authorities
- Lifecycle management experience
Visit the official Sun Pharma Careers page and search for the job title “Executive – Regulatory Affairs” to submit your application. Sun Pharma Hiring for Executive Regulatory Affairs
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.



