Apply for Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific PPD with global exposure, growth opportunities, and excellent benefits.
Introduction
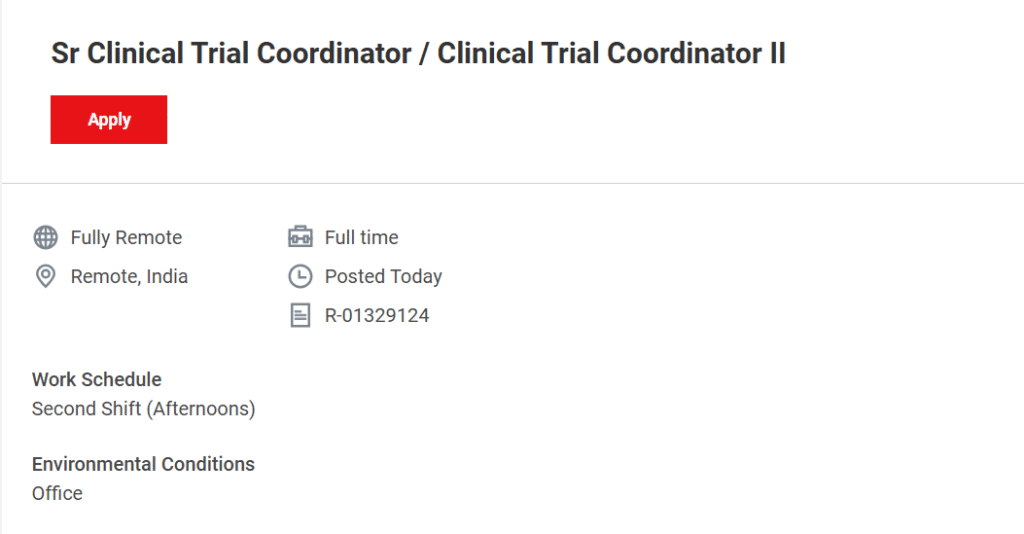
Thermo Fisher Scientific, through its global clinical research division PPD, is inviting applications for the role of Senior Clinical Trial Coordinator (Sr CTC) / Clinical Trial Coordinator II for a fully remote position in India. This opportunity is ideal for individuals looking to advance their clinical research career while working with world-class project teams engaged in life-changing clinical studies across multiple therapeutic areas. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
With a strong legacy of delivering high-quality clinical development services, PPD continues to expand its global talent pool. This role provides an opportunity to contribute to critical clinical trials, support project delivery teams, and ensure regulatory-compliant and inspection-ready study documentation. If you have experience with eTMF, CTMS, Veeva Vault, and global trial coordination, this job offers a stable, growth-oriented career path within a highly respected organization. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
Job Overview
- Role: Sr Clinical Trial Coordinator / Clinical Trial Coordinator II
- Company: Thermo Fisher Scientific – PPD
- Location: Remote, India (Work From Home)
- Job Type: Full-time
- Work Hours: Second Shift (Afternoons)
- Environment: Fully Remote Office
- Experience Required: Minimum 4+ years
- Education Required: B.Pharm, M.Pharm, Pharm.D, BSc, MSc, Life Sciences
This position is suited for detail-oriented professionals capable of managing complex study documentation, supporting global project teams, and maintaining accurate, regulatory-compliant clinical records. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
About Thermo Fisher Scientific PPD
PPD operates as part of Thermo Fisher Scientific, the world leader in serving science. With a mission focused on helping customers make the world healthier, cleaner, and safer, PPD plays a major role in clinical development. Its teams support pharmaceutical, biotech, and medical device companies by managing critical clinical trial operations that contribute to the development of new therapies and vaccines. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
Joining PPD means engaging with global teams, participating in innovative research, and building a strong career foundation in clinical operations. You will work remotely while collaborating with project managers, clinical research associates, regulatory teams, and vendors located around the world. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
Key Responsibilities
The Sr Clinical Trial Coordinator / CTC II is responsible for supporting clinical project delivery, ensuring document accuracy, and maintaining all regulatory and operational requirements across assigned studies. Major responsibilities include: Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
1. Administrative and Technical Project Support
You will handle study-related administrative tasks, coordinate documentation reviews, and support project teams in completing operational activities. Responsibilities include: Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
- Supporting project managers with study planning and coordination
- Ensuring all study documents are accurate, complete, and inspection-ready
- Facilitating regular communication within and across study teams
2. Study Documentation and eTMF Oversight
A key responsibility is maintaining the electronic Trial Master File:
- Ensuring timely updates to eTMF documents
- Performing periodic quality checks
- Supporting audits and inspection readiness
- Managing document uploads, version control, and metadata accuracy
Your role ensures regulatory compliance and alignment with ICH-GCP guidelines.
3. Study Task Coordination
You will coordinate activities listed in the project task matrix including:
- Reviewing internal, country-level, and investigator-site documentation
- Maintaining trackers and databases
- Updating project tools and global tracking systems
This helps ensure smooth progression of study activities. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
4. Regulatory Document Review and Support
You will assist regulatory teams by:
- Reviewing essential documents for compliance
- Supporting IRB/IEC submission processes
- Reconciling findings and ensuring accuracy
Consistent attention to detail and knowledge of regulatory requirements will be essential.
5. Risk-Based Monitoring (RBM) Support
You will assist RBM-related processes including:
- Supporting GoBalto and other study startup systems
- Tracking site activation progress
- Ensuring timely resolution of discrepancies
6. Vendor and Site Coordination
You will maintain close collaboration with vendors and sites by:
- Managing vendor-specific trackers
- Coordinating supply shipments
- Assisting with investigator site file (ISF) preparation
- Tracking study materials and addressing issues
Your role ensures uninterrupted study operations for all stakeholders.
7. Communication and Meeting Management
You will organize and support all project-level meetings by:
- Scheduling team calls
- Preparing agendas
- Drafting and circulating meeting minutes
- Tracking follow-up actions
Effective communication is essential for maintaining alignment across global teams. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
8. Translation and Quality Control
If required, you will support:
- Translation quality checks
- Document accuracy reviews
- Finalization of site-ready study materials
9. Onboarding and Mentoring Support
You may assist in:
- Training new team members
- Sharing best practices
- Contributing to process improvement initiatives
This fosters a culture of continuous learning within the organization.
Required Qualifications
Candidates should possess strong technical and operational capabilities relevant to global clinical trial coordination: Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
Educational Requirements
- Bachelor’s degree in Life Sciences, Pharmacy, or related field
(B.Pharm, M.Pharm, Pharm.D, BSc, MSc preferred)
Experience Requirements
- Minimum 4+ years of relevant experience as a Clinical Trial Coordinator
- Experience in global clinical trials
- Hands-on experience with:
- Veeva Vault
- CTMS systems
- eTMF management
- Study startup and documentation systems
Technical Skills
- Strong understanding of ICH-GCP and regulatory guidelines
- Proficiency in MS Office (Word, Excel, PowerPoint)
- Ability to manage multiple deadlines
- Excellent written and verbal communication
- High-quality organizational and analytical skills
Soft Skills
- Ability to work independently
- Strong teamwork and collaboration abilities
- High attention to detail
- Problem-solving skills
- Time management expertise
Why Join Thermo Fisher Scientific PPD?
Working with PPD offers significant opportunities for professional and personal growth: Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
1. 100% Remote Work Flexibility
You can work from anywhere in India with full access to PPD’s global systems and support structure.
2. Exposure to Global Clinical Operations
Collaborate with multinational project teams and develop a deeper understanding of global regulatory processes.
3. Continuous Learning and Growth
PPD invests in employee development through:
- Structured training programs
- Mentoring opportunities
- Cross-functional learning
- Career pathways in project operations or management
4. Inclusive Work Culture
Expect a supportive and collaborative environment that values diversity, teamwork, and innovation.
5. Competitive Compensation
PPD offers attractive salaries, benefits, and long-term career stability.
How to Apply
To apply for the Sr Clinical Trial Coordinator / Clinical Trial Coordinator II position:
- Visit the official Thermo Fisher Scientific PPD careers page.
- Search for the job title “Sr Clinical Trial Coordinator / Clinical Trial Coordinator II – Remote India”.
- Click on Apply Now.
- Create or log in to your candidate profile.
- Upload your updated resume and complete the online application form.
- Submit the application and monitor your email for interview details.
Make sure your resume highlights experience with eTMF, CTMS, Veeva Vault, and global trial coordination to improve your selection chances. Clinical Trial Coordinator Remote Job at Thermo Fisher Scientific
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.


