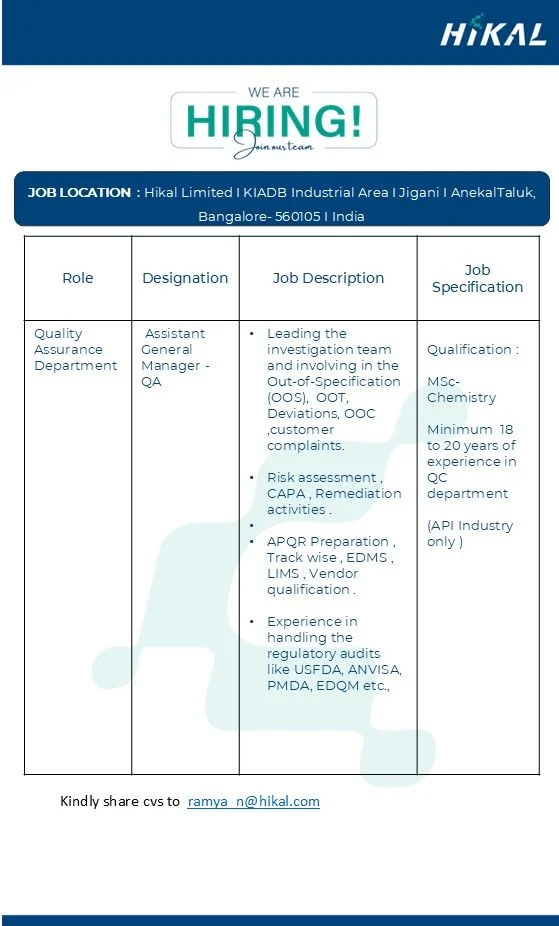
Hikal Limited is Hiring for Quality Assurance
Company: Hikal Limited
Qualification: M.Sc. Chemistry
Location: Jigani, Bangalore
Experience: 18 – 20 Years
Job Type: Full-Time
Application Mode: Email Application
Job Status: ✅ Verified Job
About the Company
Hikal Limited is a leading name in the Active Pharmaceutical Ingredient (API) manufacturing sector, known for its world-class production facilities and regulatory excellence. The company operates under strict cGMP standards and caters to global clients across regulated markets such as the USFDA, ANVISA, PMDA, and EDQM. Hikal Limited is Hiring for Quality Assurance
Job Overview
Are you an experienced Quality Assurance professional from the API pharmaceutical industry looking to take the next big step in your career?
Hikal Limited is inviting applications for the position of Assistant General Manager – QA at its state-of-the-art facility in Jigani, Bangalore. This senior leadership role is ideal for professionals with extensive QC and QA experience who can lead investigations, ensure compliance, and maintain global regulatory standards. Hikal Limited is Hiring for Quality Assurance
Key Responsibilities
- Lead and manage OOS, OOT, OOC, Deviations, and Customer Complaints investigations.
- Conduct detailed risk assessments and implement effective CAPA and remediation measures.
- Oversee APQR (Annual Product Quality Review) preparation and review processes.
- Manage QA systems such as Trackwise, EDMS, LIMS, and vendor qualification.
- Ensure compliance with international regulatory audits – USFDA, ANVISA, PMDA, EDQM.
- Supervise QA operations in a cGMP-compliant API manufacturing environment.
Required Qualifications & Experience
- M.Sc. in Chemistry (mandatory).
- Minimum 18–20 years of experience in Quality Control / Quality Assurance within the API industry.
- Strong expertise in OOS/OOT investigations, deviations, and customer complaints.
- Sound understanding of risk assessment, CAPA, and remediation activities.
- Proficiency in Trackwise, EDMS, LIMS, and related QA systems.
- Proven experience handling regulatory audits such as USFDA, ANVISA, PMDA, and EDQM.
- Excellent analytical, documentation, and leadership abilities.
Note: Only candidates from the API pharmaceutical industry will be considered for this position.
Benefits at Hikal Limited
- Competitive salary package with performance-based incentives.
- Comprehensive health insurance and employee wellness programs.
- Opportunities for career growth in a globally recognized USFDA-approved facility.
- Exposure to international quality and regulatory standards.
- Supportive work environment ensuring a healthy work-life balance.
- Hikal Limited is Hiring for Quality Assurance
How to Apply
Interested and eligible candidates can send their updated CV to: Hikal Limited is Hiring for Quality Assurance
📧 ramya.n@hikal.com
Please mention in the subject line:
👉 “Application for Assistant General Manager QA – Jigani”
Frequently Asked Questions (FAQs)
1. What experience is required for the AGM QA role at Hikal Limited?
Candidates must have 18–20 years of experience in the Quality Control / QA department within the API pharmaceutical industry, with expertise in OOS investigations and regulatory audits (USFDA, ANVISA, etc.).
2. Can freshers or non-API background candidates apply?
❌ No. Only experienced professionals from the API sector with an M.Sc. Chemistry background are eligible for this role.
Hikal Limited is Hiring for Quality Assurance
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.


