Remote Job ICON plc Hiring Site Management Associate I at global CRO ICON.
Overview
ICON plc, a world-leading healthcare intelligence and clinical research organization, is inviting applications for the position of Site Management Associate I (SMA I) in India. This exciting opportunity allows you to join a global team that is reshaping the future of clinical development through data-driven insights, operational excellence, and innovative thinking. Remote Job ICON plc Hiring Site Management Associate I
As an SMA I at ICON, you will support the management of clinical trial sites by assisting with site-level monitoring activities, ensuring compliance with global regulatory standards, and helping maintain the highest quality in research operations. This role is designed for individuals looking to start or build a strong career in the clinical research and life-sciences industry. Remote Job ICON plc Hiring Site Management Associate I
ICON plc provides a flexible, inclusive, and global work environment, enabling employees to grow professionally while contributing to healthcare innovations that positively impact millions of lives worldwide. Remote Job ICON plc Hiring Site Management Associate I
Job Details
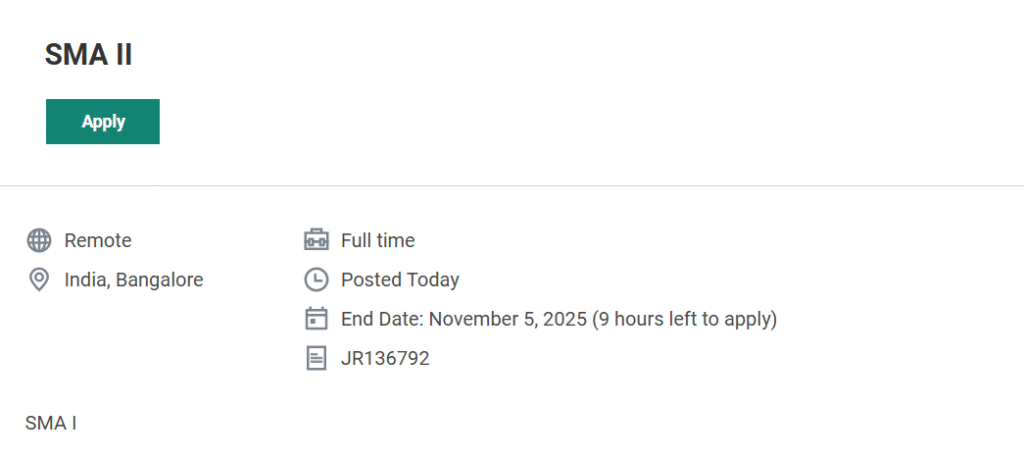
- Job Title: Site Management Associate I (SMA I)
- Job Requisition ID: JR136792
- Location: Remote – India (Bangalore preferred)
- Employment Type: Full-time
- Work Schedule: Standard (Monday to Friday)
- Last Date to Apply: November 5, 2025
About ICON plc
ICON plc is one of the world’s leading Clinical Research Organizations (CROs), offering comprehensive solutions in clinical development, data analytics, laboratory services, and regulatory support. With operations in more than 100 countries, ICON partners with major pharmaceutical, biotechnology, and medical-device companies to bring life-saving therapies to market faster and more efficiently. Remote Job ICON plc Hiring Site Management Associate I
ICON’s mission is simple yet profound — to improve the lives of patients by accelerating the development of drugs and devices that matter most. The company’s success is driven by its people, who bring dedication, innovation, and integrity to every project. Remote Job ICON plc Hiring Site Management Associate I
Position Overview
The Site Management Associate I position plays a vital role in ensuring the smooth operation of clinical trials at investigator sites. You will collaborate closely with Clinical Research Associates (CRAs), Project Managers, and other team members to ensure that each site adheres to study protocols, Good Clinical Practice (GCP), and all regulatory requirements. Remote Job ICON plc Hiring Site Management Associate I
This entry-level to early-career role is perfect for candidates with a background in life sciences, pharmacy, or healthcare administration who are passionate about advancing clinical research and ensuring compliance. Remote Job ICON plc Hiring Site Management Associate I
Key Responsibilities
As a Site Management Associate I, your primary responsibilities will include:
1. Clinical Trial Support
- Assist in monitoring clinical trial sites to ensure adherence to study protocols, GCP guidelines, and regulatory requirements.
- Coordinate and support site start-up activities, documentation collection, and essential record tracking.
- Participate in reviewing and maintaining Trial Master Files (TMFs) and other critical documents.
2. Documentation and Data Management
- Prepare and maintain study documentation such as logs, reports, and compliance checklists.
- Perform data entry, track site metrics, and ensure all data are accurate and up-to-date.
- Assist in generating and finalizing monitoring visit reports and follow-up letters.
3. Cross-Functional Collaboration
- Work closely with clinical operations, regulatory affairs, quality assurance, and project teams to ensure seamless communication and execution.
- Support issue resolution by facilitating prompt responses between sites and project stakeholders.
4. Compliance and Quality Assurance
- Ensure all site activities meet ICON’s quality standards and regulatory expectations.
- Maintain compliance with internal Standard Operating Procedures (SOPs) and external regulations (ICH-GCP, FDA, EMA, etc.).
- Participate in internal audits or inspections if required.
5. Continuous Learning and Development
- Engage in training and upskilling programs related to clinical research operations.
- Keep updated with evolving clinical research methodologies and digital tools.
- Contribute ideas for process improvement and operational efficiency.
Required Qualifications
To qualify for this role, candidates must meet the following criteria:
- Educational Qualification: Bachelor’s degree in Life Sciences, Pharmacy, Nursing, Healthcare Administration, Biotechnology, or a related field.
- Experience: Some prior exposure to clinical research, site management, or administrative coordination in healthcare is preferred but not mandatory.
- Knowledge of: Clinical trial processes, GCP principles, and regulatory frameworks.
Key Skills and Attributes
The ideal candidate will demonstrate:
- Strong organizational skills and meticulous attention to detail.
- Ability to manage multiple tasks efficiently in a fast-paced environment.
- Good understanding of clinical trial terminology and workflow.
- Excellent written and verbal communication skills in English.
- Proficiency in MS Office tools (Excel, Word, PowerPoint, Outlook).
- A proactive and collaborative attitude with a strong teamwork ethic.
- Willingness to learn, adapt, and grow within a global CRO environment.
Why Join ICON plc?
ICON plc values its employees as its greatest strength. The organization fosters an environment where everyone can thrive professionally while maintaining a healthy work-life balance. Remote Job ICON plc Hiring Site Management Associate I
Employee Benefits and Perks
ICON provides a wide range of benefits tailored to promote overall well-being and job satisfaction:
- Competitive salary packages aligned with market standards.
- Generous annual leave entitlements.
- Comprehensive health insurance coverage for employees and their families.
- Retirement and savings plans to help employees plan for the future.
- Access to a Global Employee Assistance Programme (TELUS Health) offering 24-hour professional support.
- Life assurance and financial protection plans.
- Optional benefits including childcare vouchers, discounted gym memberships, subsidised travel passes, and more.
- Global opportunities for career growth, cross-functional collaboration, and continuous learning.
Diversity and Inclusion at ICON
At ICON, diversity and belonging are core values. The company is committed to creating an inclusive workplace where all employees are treated fairly and respectfully. ICON provides equal employment opportunities without discrimination based on race, gender, religion, disability, or veteran status. Remote Job ICON plc Hiring Site Management Associate I
If you require reasonable accommodation due to a medical condition or disability during the recruitment process, ICON encourages you to request support through their official careers portal. Remote Job ICON plc Hiring Site Management Associate I
Work Environment
- Work Mode: Fully Remote (with hybrid options available)
- Location: India – Bangalore preferred
- Schedule: Monday to Friday (Standard Business Hours)
- Collaboration: Global multicultural teams, inclusive culture, and strong mentorship support.
This flexible setup enables you to balance personal and professional commitments while contributing to cutting-edge clinical research. Remote Job ICON plc Hiring Site Management Associate I
Who Should Apply?
This role is ideal for:
- Recent graduates or early-career professionals seeking entry into the clinical research industry.
- Individuals with a background in life sciences or healthcare administration who aspire to work on global clinical projects.
- Candidates who enjoy data management, documentation, and process optimization.
- Professionals who value precision, teamwork, and global impact.
Even if you don’t meet every single qualification, ICON encourages you to apply anyway — you might be the perfect fit for this role or another exciting opportunity within the organization.
How to Apply
Interested candidates can apply directly through the official ICON Careers Portal by following the steps below:
- Visit the official ICON Careers website – https://careers.iconplc.com
- In the search bar, type “Site Management Associate I” or Job ID JR136792.
- Click on the relevant job title to open the detailed description.
- Review the requirements carefully and click “Apply Now.”
- Create or log in to your ICON account, upload your updated resume, and complete the application form.
Application Deadline: November 5, 2025 (9 hours left to apply). Early applications are strongly encouraged.
Conclusion
The Site Management Associate I (SMA I) position at ICON plc offers a valuable opportunity to start or advance your career in clinical research while working with one of the world’s most respected CROs. With its flexible remote model, inclusive culture, and global reach, ICON provides the perfect platform to learn, grow, and make a real difference in healthcare. Remote Job ICON plc Hiring Site Management Associate I
If you are passionate about clinical operations, data accuracy, and medical innovation, don’t miss this chance — apply now and begin your journey toward shaping the future of clinical development with ICON plc. Remote Job ICON plc Hiring Site Management Associate I
For verified pharma job updates, visit PharmaJobHub.in – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.


